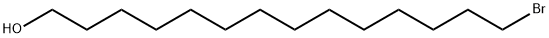General procedure for the synthesis of 14-bromo-1-tetradecanol from 1,14-tetradecanediol: 1,14-tetradecanediol (1.5 g) was dissolved in 16.5 mL of cyclohexane, to which 57% aqueous hydrobromic acid solution (16.5 mL) was added. The reaction mixture was refluxed under stirring conditions for 6 hours. After completion of the reaction, the mixture was extracted three times with ether. The organic layer was neutralized with saturated sodium bicarbonate solution and subsequently washed with brine solution and dried with magnesium sulfate. After drying, it was filtered and the solvent was removed by distillation under reduced pressure. The residue was purified by rapid chromatography on silica gel (eluent ratio: hexane:ethyl acetate = 7:3) to afford 14-bromo-1-tetradecanol as white crystals in 67% yield. Molecular weight: 292.90 (C14H29BrO).TLC (unfolding agent ratio: hexane:ethyl acetate=7:3) Rf value=0.53.1H-NMR (300 MHz, CDCl3) δ: 1.26 (s, 20H, -(CH2)10-); 1.56 (qt, 2H, J=7.0 Hz, -CH2-); 1.85 (qt , 2H, J=7.1 Hz, -CH2-); 3.40 (t, 2H, J=6.9 Hz, -CH2Br); 3.64 (t, 2H, J=6.6 Hz, -CH2O-).13C-NMR (75 MHz, CDCl3) δ: 25.72; 28.17; 28.75; 29.41-32.80; 32.82; 34.05; 63.08.