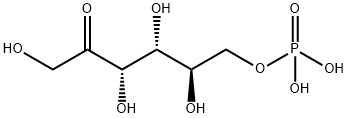
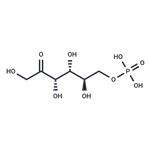
Crystallise fructose-6-phosphate as the barium salt from water by adding 4-volumes of EtOH. The barium can be removed by passage through the H+ form of a cation exchange resin, and the free acid is collected by freeze-drying. Alternatively the Ba salt is dissolved in H2O, and one equivalent of Na2SO4 is added in small portions with stirring, filter off BaSO4 and freeze dry to give the Na salt. The 6-phosphate hydrolyses more slowly than the 1-phosphate and considerably slower than pyrophosphoric acid (102 times) and triphosphoric acid (103 times). [Neuberg Biochem Zeitschrift 88 432 1918, pKa: Meyerhof & Lohmann Biochem Zeitschrift 185 113, 131 1927, Neuberg et al. Arch Biochem 3 33, 40 1944, Hydrolysis: Friess J Am Chem Soc 74 5521 1954, Beilstein 1 I 464, 1 IV 4423, H 31 537.]