Nonflamin,Yoshitomi,Japan,1971
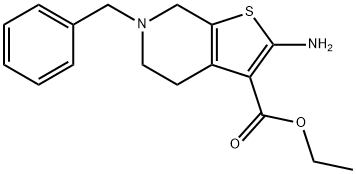
Ethyl 2-Amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylate (Tinoridine) targets pantothenate synthetase which plays a significant role in the biosynthesis of pantothenate in Mycobacterium tuberculosis (MTB).
ChEBI: Tinoridine is a thienopyridine.
A solution of 1-benzyl-4-piperidone, ethyl cyanoacetate, powdery sulfur and
morpholine in ethanol is heated moderately under reflux for about 20 minutes
to dissolve the powdery sulfur. The mixture is heated under reflux for one
further hour to complete the reaction. On standing at room temperature, the
mixture yields a precipitate. The precipitate is collected by filtration, washed
well with methanol and recrystallized from methanol to give 2-amino-6-
benzyl-3-ethoxycarbonyl-4,5,6,7-tetrahydrothieno(2,3-c)-pyridine as almost
colorless needles melting at 112° to 113°C.
Tinoridine Hydrochloride
is JAN.