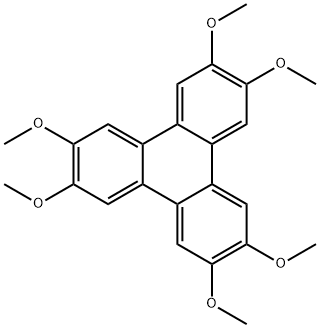
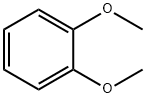
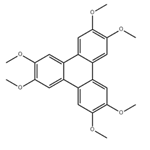
The general procedure for the synthesis of 2,3,6,7,10,11-hexamethoxytriphenylene from o-anisidine is as follows: B-type crystals of 2,3,6,7,10,11-hexahydroxytriphenylene monohydrate were first synthesized with reference to the methods reported in Synthesis, 477, 1994 and JP-A-8-119894. This was done as follows: 1,2-dimethoxybenzene (31.78 g, 0.23 mol) was co-dissolved with anhydrous ferric chloride (120 g, 0.74 mol) in 70% sulfuric acid and the reaction was stirred at 25 °C for 24 hours. After completion of the reaction, the reaction mixture was slowly poured into ice water (500 g) and the precipitated crystals were collected by filtration. Subsequently, the resulting crystals were washed with distilled water (1 L) and dried to give light purple 2,3,6,7,10,11-hexamethoxytriphenylene (28.2 g, 90.1% yield as 1,2-dimethoxybenzene). This method is derived from Synthesis, 477, 1994.