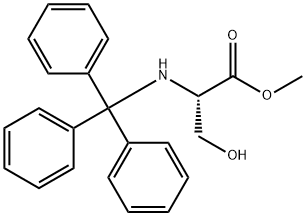
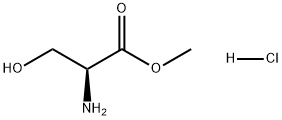
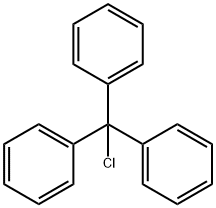
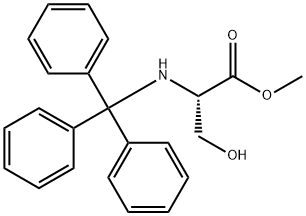
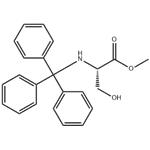
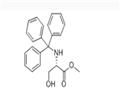
A solution of dichloromethane (CH2Cl2, 40 mL) with triethylamine (Et3N, 13.4 mL, 96.78 mmol) was slowly added dropwise to a dichloromethane (CH2Cl2, 129 mL) containing L-serine methyl ester hydrochloride (5.0 g, 32.26 mmol) and triphenylmethane chloride (Ph3CCl, 13.5 g, 48.39 mmol) solution and the reaction was carried out at 0 °C under the protection of nitrogen (N2). Subsequently, the reaction system was gradually warmed up to room temperature with continuous stirring overnight. Upon completion of the reaction, the reaction was quenched with saturated sodium bicarbonate (NaHCO3) solution and the aqueous phase was extracted with dichloromethane (CH2Cl2). The organic phases were combined, washed with brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford N-trityl-L-serine methyl ester as a colorless solid (11.41 g, 98% yield).
[1] Acta Chemica Scandinavica, 1994, vol. 48, # 6, p. 511 - 516
[2] Patent: WO2007/68474, 2007, A1. Location in patent: Page/Page column 51; 52
[3] Patent: WO2016/8946, 2016, A1. Location in patent: Page/Page column 101
[4] Angewandte Chemie - International Edition, 2017, vol. 56, # 40, p. 12245 - 12249
[5] Angew. Chem., 2017, vol. 129, p. 12413 - 12417,5