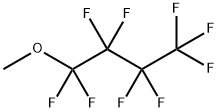
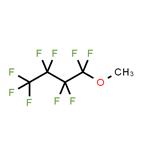
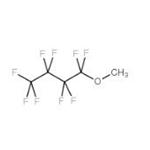
Methyl Perfluorobutyl Ether is a clear, colorless, low-odor, non-toxic, non-corrosive, non-flammable liquid substance with a chemical formula of C5H3F9O and a molecular weight of 250.06. Since it has low flammability, relatively low vapor pressure, low heat of vaporization, and low surface tension, it is considered to replace the chlorine-containing cleaning solvents such as 1,1,1-trichloroethane, CFC-113 (CF2ClCFCl2), and HCFC-141b (CH3CFCl2). Besides, it is a fluorinated ether solvent that is very compatible with many other ingredients, and is thought to have good sliding properties and serve as a good solvent in oil-based formulas.
Because methyl perfluorobutyl ether contains perfluoroalkyl group (Rf) on one side and alkyl group (R) on the other side of ether linkage, it cannot be synthesized by selective partial fluorination of its hydrocarbon precursors. Instead, they may be prepared by coupling reactions of two reactants having Rf and R, coupling reactions of two reactants having Rf and R, respectively.
Nonafluorobutyl methyl ether (Methyl Nonafluorobutyl Ether, MFE) may be used to compose mixed-solvent electrolytes. Flash point, conductivity and NMR spectra of these solvent mixtures have been investigated.