It was realized in 1928 that the administration of teleost pituitary gland extract led to concentrated pigment in
fish scales. In 1983, Kawauchi purified and sequenced the
factor, named melanin-concentrating hormone (MCH),
for the first time from the salmon pituitary. In mammals,
the primary structure of rat MCH was determined by
purification methods in 1989, and that of the human
was determined from the cDNA sequence in 1990.
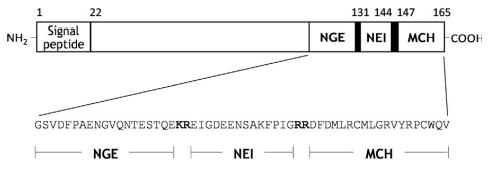
Human proMCH consists of 165 aa residues with
mature MCH at the C-terminus. Mature MCH is cleaved
at the KR sequence either by prohormone convertase
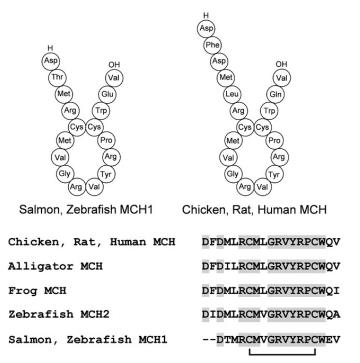
PC1/3 or PC2. MCH is a cyclic peptide formed by a disulfide bridge, and comprises 19 aa residues in tetrapods
and 17 aa residues (MCH1) or 19–25 aa residues
(MCH2) in teleost fish. The primary structure is well conserved; human MCH
is identical to that of rodents and is highly similar to that
of teleost fish. The central ring sequence between two cysteines is the most significant factor for melanin concentrating activity. Mr 2386.8; pI >9.5 (mouse, rat, human MCH). MCH is
freely soluble in water, ethanol, and 70% acetone; insoluble in acetone, benzene, chloroform, and ether.
Gene, mRNA, and precursor
The human MCH gene (PMCH), located on chromosome 12 (12q 23-q24), consists of three exons.3 Exon 2
encodes neuropeptide G-E (NGE) and neuropeptide E-I
(NEI), and exons 2 and 3 encode MCH.
Two variant PMCH genes are also identified on 5p14
(PMCHL1) and 5q12-q13 (PMCHL2), but only a single
locus is found in the rodent species. Teleost MCH2 is the ortholog of mammalian MCH, and MCH1 is a highly
conserved paralog of MCH2. The MCH2 gene might be
silent in some teleost fish such as salmonids.
In rodents, MCH neurons receive projections from
many brain areas and become active during REM sleep
and novel object exploration. Neurotransmitters such
as GABA, serotonin, acetylcholine, noradrenaline, dopamine, and neuropeptide Y all inhibit the MCH neurons.
The firing of orexin neurons also inhibits MCH neurons
via microcircuits. The metabolic factors glucose and insulin can enhance MCH neuronal activity, although glucose
exerts the opposite effect on the orexin neurons. MCH
may be coreleased with glutamate, GABA, or other neuropeptides and act synergistically. The activation of
hypothalamic MCH neuron terminals reduced the firing
of hippocampal pyramidal neurons by increasing the
inhibitory inputs.
MCH acts via two G protein-coupled receptors,
MCHR1 and MCHR2, of which functional MCHR2 is
not present in rodents. MCHR1 is a glycoprotein consisting of 353 aa residues in the rat/human and maps to chromosome 22, q13.3 in humans. MCH binds to rat and
human MCHR1s with Kd values of 1.6 and 0.5 nM,
respectively. Strong labeling of MCHR1 mRNA is
detected in several limbic structures and anatomical areas
implicated in the control of olfaction in rats. MCHR1 is
observed to be selectively targeted to a hair-like organelle
named the primary cilium on the rodent and human neuronal cell. Human MCHR2 is also highly expressed in
the brain.
Signal transduction pathway
In recombinant cell lines, rat, mouse, and human
MCHR1s are promiscuous, and couple to various GTP
binding proteins such as Gi, Go, and Gq proteins. In contrast to human MCHR1, however, human MCHR2 exclusively couples to the Gq protein.
The transgenic mice overexpressing the MCH gene are
obese and insulin-resistant. Mice lacking the MCH gene
are hypophagic, hyperactive, and lean. The MCH gene is
upregulated in the leptin-deficient ob/ob mice.
MCHR1-/- mice are hyperphagic but lean because of
hyperactivity and altered metabolism. REM sleep-active
MCH neurons in the hypothalamus are involved in active
forgetting in the hippocampus. In addition, mood-,
memory-, and sleep-related phenotypes are also
reported.
So far, no hereditary diseases have been associated
with MCH, MCHR1, or MCHR2 genes. The association
of obesity with SNP in PMCH, MCH1R, and MCH2R
has been investigated, but the results were not consistent.
A case having autoimmunity to MCHR1 is reported to
have developed vitiligo. In an orexin-deficient mouse
model of narcolepsy, the MCH-MCHR1 system contributes to abnormal REM sleep regulation manifest as
cataplexy.
Melanin-concentrating hormone is an orexigenic peptide
produced in the lateral hypothalamic area and zona incerta
of the mammalian brain. MCH neurons constitute a powerful regulatory system with wide and divergent projection,
associated with food intake, energy expenditure, mood,
REM sleep, and memory.
Melanin Concentrating Hormone, salmon is a 19-amino-acid neuropeptide initially identified in the pituitary gland of teleost fish, which regulates food intake, energy balance, sleep state, and the cardiovascular system. Melanin-concentrating hormone is a ligand for an orphan G protein-coupled receptor (SLC-1/GPR24) and MCHR2.
Melanin Concentrating Hormone stimulates appetite. Continuous infusion of Melanin Concentrating Hormone into the ventricular system increases food intake for 7-8 days[2]. Intracerebroventricular infusion of Melanin Concentrating Hormone (10 μg/day) causes a slight but significant increase in body weight in mice maintained on the regular diet. Chronic stimulation of the brain Melanin Concentrating Hormone system could cause obesity in mice[3].
[1] An S, et al. Identification and characterization of a melanin-concentrating hormone receptor. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7576-81. DOI:
10.1073/pnas.131200698[2] Della-Zuana O, et al. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord. 2002 Oct;26(10):1289-95. DOI:
10.1038/sj.ijo.0802079[3] Gomori A, et al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone. Am J Physiol Endocrinol Metab. 2003 Mar;284(3):E583-8. Epub 2002 Nov 26. DOI:
10.1152/ajpendo.00350.2002