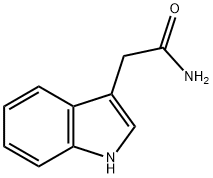
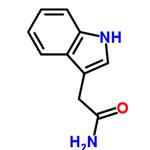
Indole-3-acetamide was used in the synthesis of [5.5.6.6]diazafenestrane skeleton and indole-3-acetic acid.
Reactant for the synthesis of:
PET agent for imaging of protein kinase C
A potential agent against Prion Disease
Protein kinase C (PKC) inhibitor bisindolylmaleimide IV
Glycogen synthase kinase-3 (GSK-3) inhibitors
Inhibitors of CaMKIId
A VEGF inhibitor
JAK3 inhibitors
Inhibitors of NAD+-Dependent Histone Deacetylases
Inhibitors of human adipocyte fatty acid-binding protein
Cyclin-dependent kinase inhibitors
ChEBI: Indole-3-acetamide is a member of the class of indoles that is acetamide substituted by a 1H-indol-3-yl group at position 2. It is an intermediate in the production of plant hormone indole acetic acid (IAA). It has a role as a fungal metabolite, a bacterial metabolite and a plant metabolite. It is a N-acylammonia, a monocarboxylic acid amide and a member of indoles. It is functionally related to an acetamide.
The general procedure for the synthesis of 3-indoleacetamide from indole-3-acetonitrile was as follows: the reaction was carried out in a 100-mL stainless steel autoclave. Indole-3-acetonitrile (0.10 g, 1.00 mmol) was added to a mixture of ethylenediamine (0.12 g, 2.00 mmol), CoCl2-H2O (0.24 g, 1.00 mmol), and Zn (0.07 g, 1.00 mmol) and the mixture was transferred to the autoclave. For reactions to be performed in solvent, an additional 5 mL of solvent was added. The autoclave was pressurized to 5.00 atm using O2. the reaction mixture was stirred in a preheated 120°C oil bath for 12 hr. Upon completion of the reaction, the mixture was cooled to room temperature and the product was purified by silica gel column chromatography using a solvent mixture of n-hexane and CH2Cl2 (1:1) as eluent to give the final 3-indoleacetamide in 88% yield.
[1] Tetrahedron, 1989, vol. 45, # 5, p. 1347 - 1354
[2] Research on Chemical Intermediates, 2015, vol. 41, # 8, p. 5071 - 5078
[3] Chemistry of Heterocyclic Compounds (New York, NY, United States), 1980, vol. 16, # 5, p. 501 - 506
[4] Khimiya Geterotsiklicheskikh Soedinenii, 1980, # 5, p. 645 - 650