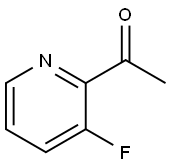
General procedure for the synthesis of 3-methyl-1H-pyrazolo[4,3-b]pyridine from 2-acetyl-3-fluoropyridine: 1-(3-fluoropyridin-2-yl)ethanone (1.00 g, 7.19 mmol) was dissolved in hydrazine monohydrate (12 mL, 247.00 mmol), which was heated in a microwave reactor for 3 hours at 130 °C. Upon completion of the reaction, water (50 mL) was added to dilute the reaction mixture and extracted with ethyl acetate (3 x 25 mL). The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Purification by a Biotage fast chromatography system (using a SNAP 50g silica gel column with ethyl acetate/ethanol as eluent, with the ratio tapered from 95/5 to 70/30) gave a cream-colored solid product (575 mg, 60% yield). The product was identified by 1H NMR (500 MHz, DMSO-d6): δ 12.87 (broad single peak, 1H), 8.46 (double peak, J = 4.3, 1.4 Hz, 1H), 7.91 (double peak, J = 8.5, 1.4 Hz, 1H), 7.33 (double peak, J = 8.5, 4.3 Hz, 1H), 2.53 (single peak, 3H). 13C NMR (126 MHz, DMSO-d6) δ 144.51, 142.04, 139.94, 133.42, 121.12, 118.49, 11.24. LCMS (Method M) retention time 0.52 min, [M + H]+ 134; HRMS calculated value C7H8N3: 134.0718, measured value: 134.0726.
![1H-Pyrazolo[4,3-b]pyridine,3-methyl-(9CI) Structure](/CAS/GIF/194278-45-0.gif)