-325 mesh 10μm or less with 99.5% purity; refractory material [KIR78] [CER91]
Strontium boride appears as a crystalline black
powder. Closer examination reveals slightly translucent
dark red crystals capable of scratching quartz. It is very
stable and has a high melting point and density.
Although not thought to be toxic, it is an irritant to the
skin, eyes, and respiratory tract. It has the formula of
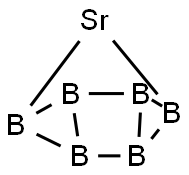
SrB6, with a molecular weight of 152.49 g/mol. It is
a black crystalline solid (15.0 °C) with a density of
3.39 g/cm3, and a melting point of 2235.0°C.
Strontium boride, along with other alkali earth metal
borides, has been shown to exhibit weak ferromagnetism
at low temperatures.
Strontium boride is used in insulation
and nuclear control rods.
Strontium boride can be formed directly from the
elements. Sr melts at 777°C and boron melts at
2076 °C. Therefore, if a vapor of Sr metal at >850°C
(red-heat) is passed over crystals of boron, reaction
forms the desired boride. However, to obtain stoichiometric
compositions, it is better to heat the well-mixed
powders of Sr and B to obtain specific compounds:
Sr+ 6B→SrB6