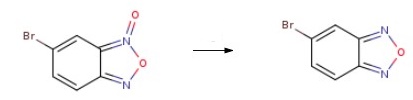
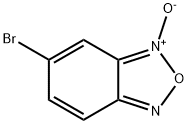
General procedure for the synthesis of 5-bromo-2,1,3-benzoxazole from 5-bromobenzo[2,1,3]oxadiazole 3-oxide: triphenylphosphine (1.1 mmol) was dissolved in anhydrous toluene (3 mL) and stirred at 110 °C under argon protection. Subsequently, a solution of 6-bromobenzo[c][1,2,5]oxadiazole 1-oxide (1 mmol) in toluene (0.5 mL) was slowly added dropwise over a period of 1 h. Stirring of the reaction mixture was continued for 3 h after completion of the dropwise addition. After completion of the reaction, the solvent was removed by distillation under reduced pressure and the crude product was purified by fast column chromatography (eluent: cyclohexane-ether, 98:2) to afford the target compound 5-bromo-2,1,3-benzoxazole as a light pink solid. Yield: 60%. Thin layer chromatography (TLC) conditions (cyclohexane-ether, 98:2): rf = 0.5. melting point: 74 °C. 1H-NMR (300 MHz, DMSO-d6) δ: 8.49 (s, 1H, H4), 8.04 (d, J = 9.4 Hz, 1H, H7), 7.69 (dd, J = 9.4, 1.6 Hz, 1H, H6).
![5-Bromobenzo[c][1,2,5]oxadiazole Structure](/CAS/GIF/51376-06-8.gif)
![5-Bromobenzo[c][1,2,5]oxadiazole pictures](/ProductImageEN/2024-07/Small/45352336-ff89-4f9d-9f7d-f0d29e66ca70.jpg)