Sodium azide
26628-22-8 Basic informationMore
- Product Name:Sodium azide
- Synonyms: Sodium azide,azium Sodium azide, extra pure, 99% SODIUM AZIDE REAGENT (ACS) SodiuM azide, 99+%, for biocheMistry azoturedesodium nci-c06462 nemazyd nsc3072
- CAS:26628-22-8
- MF:N3Na
- MW:65.01
- EINECS:247-852-1
- Mol File:26628-22-8.mol
-
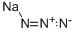
USE
<strong>Biomedical Sciences</strong><br />
Sodium azide is a biocide that is used in making chemical preservatives that are used in laboratories and hospitals. In hospitals, it is used as a preservative in blood tests and diagnostic machines. Since the chemical is soluble, it can act as useful metabolic inhibitors that can affect the activities of numerous oxidative enzymes. Specifically, it inhibits oxidative enzymes that are involved in electron transport system respiration. However, accidents have occurred whenever sodium azide is used in a hospital setting; therefore, major bodies such as the CDC that deal with chemicals do not advise the use of the compound.<br />
<strong>Automobiles Airbags</strong><br />
Before the 1990s, sodium azide was used in airbags. In airbags, when electricity heats sodium azide to approximately 300oC, it decomposes into two simple components, namely sodium and nitrogen gas. The decomposition of sodium azide happens very fast that the filling of the airbag with nitrogen gas occurs at a velocity of around 200 miles per hour.<br />
<strong>Agriculture</strong><br />
Sodium azide is used for pest control due to its ready solubility in water. Nitrifying bacteria acts as a catalyst that decomposes the sodium azide solution to release nitrate, HN3, and NH4+ when added to the soil. It is noteworthy that these components have wide range of activity against nematodes, weeds, and phytopathogenic fungi that is soil-borne. The chemical is typically applied through drip irrigation to avoid reactivity of HN3 in the soil-air space that can eventually result in an active compound that can be ineffective for pest control.<br />
<strong>Detonators</strong><br />
When combined with other metals such as copper (Cu), lead (Pb), and mercury (Hg), sodium azide becomes unstable and explosive. As such, it is mostly used as detonators due to its explosive nature.<br />
A summary of main uses is listed in the table below:
<table align="center" border="1" bordercolor="#ccc" cellpadding="5" cellspacing="0" style="border-collapse:collapse">
<tbody>
<tr>
<td style="width:100px"><strong>Industry</strong></td>
<td style="width:200px"><strong>Application</strong></td>
<td><strong>Role/benefit</strong></td>
</tr>
<tr>
<td rowspan="2">Chemical manufacture</td>
<td>Preparation of other inorganic azide compounds, such as lead azide and silver azide</td>
<td>Precursor</td>
</tr>
<tr>
<td>Preparation of organic azide compounds</td>
<td>Azidation agent</td>
</tr>
<tr>
<td rowspan="2">Agriculture</td>
<td>Pest control of soil-borne pathogens</td>
<td>Biocide</td>
</tr>
<tr>
<td>Crop selection of plants such as rice,barley or oats</td>
<td>Mutagen</td>
</tr>
<tr>
<td>Pharmaceutical</td>
<td>Preparation of tetrazoles pharmaceutical intermediates</td>
<td>Raw material</td>
</tr>
<tr>
<td>Airbags</td>
<td>Automobile airbags and aircraft safety chutes</td>
<td>Gas source/generates n2 after automobile crash</td>
</tr>
<tr>
<td rowspan="2">Antisepsis and sterilization</td>
<td>Preservation of bulk reagents and stock solutions in hospitals and laboratories</td>
<td>Biocide/inhibits the cytochrome oxidase in gram-negative bacteria</td>
</tr>
<tr>
<td>Photographic emulsion</td>
<td>Preservative/works without influencing the performance of photographic emulsion</td>
</tr>
<tr>
<td>Explosive</td>
<td>Manufacture of detonating agent(lead azide)</td>
<td>Raw material</td>
</tr>
<tr>
<td rowspan="2">Others</td>
<td>Manufacture of synthetic resin</td>
<td>Foaming agent/generates N<sub>2</sub> during the reaction</td>
</tr>
<tr>
<td>Manufacture of Na</td>
<td>Raw material/2NaN<sub>3</sub>→2Na+3N<sub>2</sub></td>
</tr>
</tbody>
</table>
<div> </div>
Lastest Price from Sodium azide manufacturers
Browse by Nationality 26628-22-8 Suppliers >Global suppliers
Please select the suppliers
Recommend You Select Member Companies
Recommend You Select Member Companies