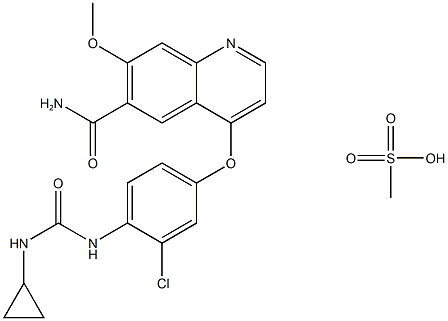
Lenvatinib mesylate (lenvatinib) is an oral targeted therapy medicine known as a receptor‐type tyrosine kinase inhibitor. It is a small-molecule drug (a drug that can enter cells easily), which was developed at Eisai in 2015. It was approved by the FDA in 2015 for the treatment of differentiated thyroid cancer that is either locally recurrent, metastatic, or progressive and did not respond to radioactive iodine treatment. In May 2016, the FDA approved the drug as a combination therapy with everolimus for the treatment of advanced renal cell carcinoma. Because VEGF (and fibroblast growth factor receptors, known as FGFRs) are thought to play a role in cardiovascular signaling pathways, VEGF2R and FGFR inhibition are thought to be the mechanisms behind the primary side effect of lenvatinib mesylate, which is hypertension.