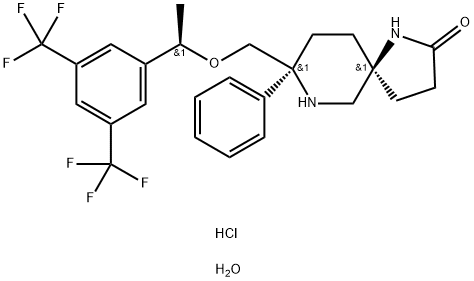
Rolapitant hydrochloride hydrate, originally discovered by
Schering-Plough and later developed by TESARO, Inc., was
approved by the FDA in September 2015 for the prevention of
delayed chemotherapy-induced nausea and vomiting (CINV)
in combination with other antiemetic agents. Rolapitant is a
highly selective NK-1 receptor antagonist, exhibiting >1000-
fold selectivity for NK-1 over human NK-2 and NK-3 receptors
in vitro. In contrast to other NK-1 inhibitors that play an
essential role in delayed CINV therapy, rolapitant shows no
inhibition of CYP3A4, eliminating the need for concern when
coadministering with CYP34A substrates. Additionally, rolapitant
is an orally active agent with a relatively long half-life (180
h), providing potential opportunities for single- and
prechemotherapy-based treatments. In three large clinical
trials involving patients receiving moderately emetogenic
chemotherapy (MEC) and highly emetogenic chemotherapy
(HEC), subjects using rolapitant as a cotherapy with
granisetron and dexamethasone showed a significant improvement
in complete response compared to those receiving
treatments of granisetron and dexamethasone.