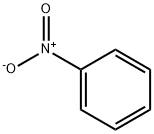
Nitrobenzene (chemical formula: C6H4NO2) is a yellowish, oily, aromatic nitro-compound. The most important application of nitrobenzene (consuming 95%) is for the manufacturing of aniline, which is an important industrial precursor. Besides aniline, it can also be used to generate related derivatives such as azobenzene, nitrosobenzene and phenylhydroxylamine. Moreover, it can be used for the production of lubricating oils, dyes, drugs, pesticides, and synthetic rubber. Another special application of it is masking unpleasant odors emitting from shoe, floor polisher, and leather as well as paint solvents. In addition, it can sometime used as a solvent, especially for electrophilic reagents in the laboratory. The nitrobenzene is mainly manufactured through the nitration of benzene with the mixture of concentrated sulfuric acid, water and nitric acid. However, the reaction process is quite dangerous due to the exothermicity of the reaction.