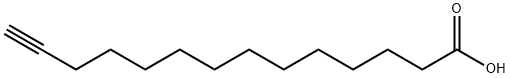
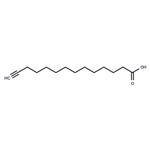
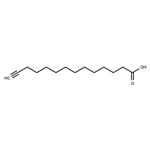
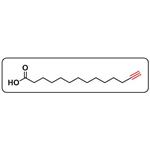
Myristic acid is a 14-carbon saturated (14:0) fatty acid. In vivo, it is commonly added covalently to the N-terminus of proteins in a co-translational process termed N-myristoylation. The sirtuin SIRT6 removes this acyl group from myristoylated TNF-α, enhancing secretion. Myristic acid alkyne is a form of this myristic acid with an ω-terminal alkyne. Such terminal alkyne groups can be used in linking reactions, known as click chemistry, characterized by high dependability and specificity of azide-alkyne bioconjugation reactions. Click chemistry has only recently been applied to the study of lipids.