-
ブランド less more
富士フイルム和光純薬株式会社(wako) -
パッケージ less more
2g
| 生産者 | 包装単位 | 価格 | 製品説明 | |
|---|---|---|---|---|
|
富士フイルム和光純薬株式会社(wako) W01USP1374292 |
2g | ¥46600 | Magnesium Phosphate (AS) |
プロパティ
| 融点 | 1184 °C |
| 比重(密度) | 503.3[at 20℃] |
| 溶解性 | soluble in dilute acid solutions |
| 色 | white crystals, crystalline |
| 臭い (Odor) | at 100.00%. odorless |
| 水溶解度 | insoluble H2O; soluble dilute mineral acids [MER06] |
| 化粧品成分の機能 | ANTICAKING BULKING OPACIFYING |
| 美容成分審査 | MAGNESIUM PHOSPHATE (7757-87-1) |
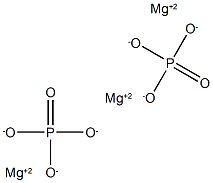
| InChI | InChI=1S/3Mg.2H3O4P/c;;;2*1-5(2,3)4/h;;;2*(H3,1,2,3,4)/q3*+2;;/p-6 |
| InChIKey | GVALZJMUIHGIMD-UHFFFAOYSA-H |
| SMILES | P(=O)([O-])([O-])[O-].P(=O)([O-])([O-])[O-].[Mg+2].[Mg+2].[Mg+2] |
| LogP | -2.148 (est) |
| CAS データベース | 7757-87-1 |
| EPAの化学物質情報 | Trimagnesium phosphate (7757-87-1) |
説明
Magnesium phosphate includes both magnesium phosphate, dibasic, and magnesium phosphate, tribasic. Magnesium phosphate, dibasic (MgHPO4.3H2O, CAS Reg. No. 7782-75-4) occurs naturally as the white, crystalline mineral newberyite. It is prepared commercially as a precipitate formed by treating a solution of magnesium sulfate with disodium phosphate under controlled conditions. Magnesium phosphate, tribasic (Mg3(PO4)2.xH2O, CAS Reg. No. 7757-87-1) may contain 4, 5, or 8 molecules of water of hydration. It is produced as a precipitate from a solution of magnesite with phosphoric acid. Magnesium plays a vital role in regulating the neuromuscular activity of the heart, converts blood sugar to energy and is necessary for proper calcium and Vitamin C metabolism. Magnesium Phosphate can be used as a dietary supplement and as a nutrient.
供給者とメーカー
- Jiangsu Kolod Food Ingredients Co.,Ltd.
- Hebei Yanxi Chemical Co., Ltd.
- Hebei Chuanghai Biotechnology Co., Ltd
- Hebei Zhuanglai Chemical Trading Co Ltd
- career henan chemical co
- Shaanxi Dideu Medichem Co. Ltd
- Henan Tianfu Chemical Co.,Ltd.
- Hubei xin bonus chemical co. LTD
- Neostar United (Changzhou) Industrial Co., Ltd.
- Hefei TNJ Chemical Industry Co.,Ltd.