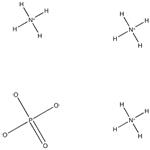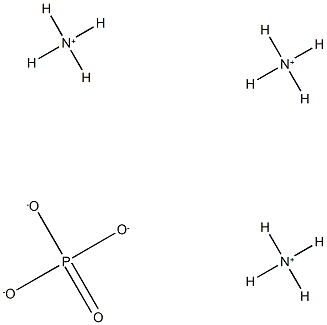
ポリリン酸アンモニウム
¥53700
化学名ポリリン酸アンモニウム
英語名Ammonium polyphosphate
CAS番号68333-79-9
分子式H12N3O4P
分子量149.086741
EINECS269-789-9
MDL NumberMFCD00241367
别名
ポリリン酸アンモニウム
抗XPNPEP3抗体 ウサギ宿主抗体
-
ブランド less more
Sigma-Aldrich Japan -
パッケージ less more
100ul
| 生産者 | 包装単位 | 価格 | 製品説明 | |
|---|---|---|---|---|
|
Sigma-Aldrich Japan SAB2700881 |
100ul | ¥53700 | 抗XPNPEP3抗体 ウサギ宿主抗体 |
プロパティ
| 比重(密度) | 1.74[at 20℃] |
| 蒸気圧 | 0.076Pa at 20℃ |
| 貯蔵温度 | −20°C |
| 溶解性 | Aqueous Acid (Slightly) |
| 外見 | Solid |
| 色 | White to Off-White |
| 由来生物 | rabbit |
| InChI | InChI=1S/3H3N.H3O4P/c;;;1-5(2,3)4/h3*1H3;(H3,1,2,3,4) |
| InChIKey | ZRIUUUJAJJNDSS-UHFFFAOYSA-N |
| SMILES | P(=O)([O-])([O-])[O-].[N+]([H])([H])([H])[H].[N+]([H])([H])([H])[H].[N+]([H])([H])([H])[H] |
| LogP | -2.148 (est) |
| CAS データベース | 68333-79-9 |
| EPAの化学物質情報 | Ammonium polyphosphates (68333-79-9) |
安全情報
-
絵表示(GHS)

-
注意喚起語
Warning
-
危険有害性情報
H302:飲み込むと有害
H319:強い眼刺激
-
注意書き
P264:取扱い後は皮膚をよく洗うこと。
P264:取扱い後は手や顔をよく洗うこと。
P270:この製品を使用する時に、飲食または喫煙をしないこ と。
P280:保護手袋/保護衣/保護眼鏡/保護面を着用するこ と。
P301+P312:飲み込んだ場合:気分が悪い時は医師に連絡する こと。
P305+P351+P338:眼に入った場合:水で数分間注意深く洗うこと。次にコ ンタクトレンズを着用していて容易に外せる場合は外す こと。その後も洗浄を続けること。
P330:口をすすぐこと。
P501:内容物/容器を...に廃棄すること。
説明
Ammonium polyphosphate (APP) is an organic salt of polyphosphoric acid and ammonia. As a chemical, it is non-toxic, environmentally friendly and halogen-free. It is most commonly used as a flame retardant, selection of the specific grade of ammonium polyphosphate can be determined by the solubility, Phosphorus content, chain length and polymerization degree. The chain length (n) of this polymeric compound can be linear or branched. Depending on the polymerization degree, there are two main families of ammonium polyphosphate: Crystal phase I APP (or APP I), and Crystal phase II APP (or APP II).
- APP phase I has a short and linear chain (n < 100), it is more water sensitive (hydrolysis) and less thermally stable; actually it begins to decompose at temperatures above 150 °C.
- The second family of Ammonium polyphosphate is the APP Phase II; which has an high polymerization degree, with n>1000, its structure is cross linked (branched), and it is an high-quality non-halogenated flame retardant. APP phase II, Ammonium polyphosphate, has an higher thermal stability (the decomposition starts at approximately 300°C) and lower water solubility than APP I.
| Appearance | White free-flowing powder |
| Whiteness | 92.0 Min |
| pH (10% slurry -25°C) | 5.5-7.5 |
| Acid Value, KOH mg/1g | 1.0 Max |
| Solubility in water (25°C), g/100ml H2O | 0.50 Max |
| Nitrogen, w/w% | 14.0-15.0 |
| Phosphorus (P), w/w% | 31.0-32.0 |
| Thermal decomposition onset, °C | 285 Min |
| Average Particle Size, D50, µm | About 15 |
供給者とメーカー
- airuikechemical co., ltd.
- Hebei Chuanghai Biotechnology Co,.LTD
- Hebei Chuanghai Biotechnology Co., Ltd
- Hebei Jingbo New Material Technology Co., Ltd
- Henan Fengda Chemical Co., Ltd
- Shandong Juchuang Chemical Co., LTD
- Hebei Zhuanglai Chemical Trading Co.,Ltd
- Hebei Longbang Technology Co., LTD
- Shandong Deshang Chemical Co., Ltd.
- HebeiShuoshengImportandExportco.,Ltd