- HOME
- Suppliers by country
- Vendor type
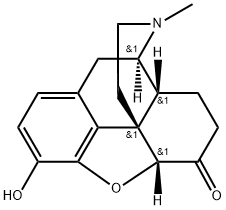
HYDROMORPHONE suppliers
- CAS:
- 466-99-9
- MF:
- C17H19NO3
- MW:
- 285.34
Properties
- Melting point::
- 266-267°
- alpha :
- D25 -194° (c = 0.98 in dioxane)
- Boiling point::
- 427.77°C (rough estimate)
- Density :
- 1.0864 (rough estimate)
- refractive index :
- 1.5400 (estimate)
- Flash point::
- 9℃
- storage temp. :
- -20°C
- solubility :
- Chloroform (Slightly), Dioxane (Slightly, Sonicated)
- form :
- Solid
- pka:
- pKa 8.15 (Uncertain)
- color :
- White to Off-White
- Water Solubility :
- 1.931g/L(25 ºC)
SecurityInformation
- Symbol(GHS) :
-



GHS02,GHS06,GHS08
- Signal word :
- Danger
- Hazard statements :
- H225-H301+H311+H331-H370
- Precautionary statements :
- P210-P260-P280-P301+P310-P311
- Hazard Codes :
- F,T
- Risk Statements :
- 11-23/24/25-39/23/24/25
- Safety Statements :
- 7-16-36/37-45
- RIDADR :
- 1544
- WGK Germany :
- 1
- HazardClass :
- 6.1(a)
- PackingGroup :
- II
- Use:
- Hydromorphone, (Dilaudid) is a synthetic derivative ofmorphine prepared by the catalytic hydrogenation and dehydrogenationof morphine under acidic conditions, using alarge excess of platinum or palladium. Oxidation of the 6-OH of morphine resulted in a compound with decreased potency.Reducing the 7,8 double bond of morphine increasedthe flexibility of the molecule and resulted in a compoundwith slightly enhanced binding. Making both of these structuralchanges to morphine-produced hydromorphone, acompound approximately 5 times as potent as morphine.Hydromorphone was introduced in 1926 and is available as animmediate release tablet, a liquid, and a suppository. A sustainedrelease form is available in some countries but not inthe United States. The sustained release form was removedfrom the U.S. market in 2005 when studies showed that drinking8 oz of alcohol (40%) could cause the drug to be releasedfrom the capsule immediately and lead to concentrations thatwere 5.5 times higher than in patients that did not drink alcohol.This potentially lethal combination prompted the Foodand Drug Administration (FDA) to remove it from the market.
More▼
▲
Your search"466-99-9"did not match any products
You may consider to:
1、Check the spelling.
2、Use less keywords.
3、Use different keywords.