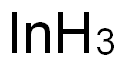-
外観
銀色~銀灰色, 粉末又は塊
-
性質
物理的性質は、帯青白色または銀灰色をしており、ナイフで切断できるほど柔らかいです。常温の空気中では非常に安定しています。化学的性質は、酸に侵されやすいですが、アルカリには安定しています。
インジウムの化合物として、酸化インジウム、リン化インジウム、ヒ化インジウム、アンチモン化インジウムなどが挙げられます。また、インジウムは質量数が113のものと115のものが存在し、113のものが安定同位体で、115のものが放射性同位体となっています。
しかし、インジウムは自然界においては質量数115のものがおよそ95%の存在比を占めており、安定同位体よりも放射性同位体の方が多くの存在比を持つという珍しい元素です。しかし、この質量数115の放射性同位体は半減期が441兆年と非常に長く、ほとんど安定同位体と言っても差し支えありません。
そのため、さまざまな電子部品にインジウムは使用されていますが、その放射能が問題とされることはありません。
-
溶解性
水に不溶。酸に易溶。アルカリに不溶。水およびアルカリ水溶液に対して安定。酸水溶液にはH2を発生して溶けIn(3+)を生じる。熱塩酸に溶ける。
-
解説
インジウム,In.原子番号49の元素.電子配置[Kr]4d105s25p1の周期表13族金属元素.元素名は原子輝線スペクトルのインジゴ色から命名された.軟らかい銀白色の金属.融点156.61 ℃,沸点2080 ℃.密度7.31 g cm-3(20 ℃).標準電極電位 In3+/In-0.3382 V.第一イオン化エネルギー5.786 eV.化学的にはアルカリ溶液に対しては抵抗を示すが,酸には容易に侵される.化合物は InⅠと InⅢ化合物があるが,InⅠ化合物は強い還元作用をもつ.インジウムはあざやかな藍色(インジゴ)の炎色反応を示し,分析には波長451.155,410.195 nm のスペクトル輝線が用いられる.
森北出版「化学辞典(第2版)
-
主な性質
- インジウムは柔らかい銀白色の金属であり、空気中では安定に存在(酸化膜ができる)。水とも反応しない
- インジウムは融点が約166℃と低いので単体金属としての用途は殆んどない
-
用途
標準液調製原料、低融点合金材料、半導体材料。
-
用途
高純度金属、合金材料、電子材料。
-
危険性
インジウムから作られるITOについて、以前に間質性肺炎による死亡例が報告されており、ITOを取り扱う作業者の間で間質性肺炎の罹患例はいくつか報告されています。それを受けて、2010年に厚生労働省からインジウム・スズ酸化物等取扱い作業による健康障害防止対策が発表されました。
-
主な用途
- 低融点合金(インジウム入りハンダヒューズ)
- 接点材料
- 蛍光体(モノクロブラウン管)
- 透明電極(液晶テレビ、太陽電池)
- 撮像管(ターゲットリング)
- 歯科合金
- ベアリング(航空機、自動車)
- 半導体素子(半導体レーザー、赤外線探知機)
- ボンディング剤(スパッタリング?ターゲット用接着剤)
- 情報記録材料(光ディスク)
-
使用上の注意
純度は金属ベースで差数法によって算出したもので、重量又は容量分析等の化学的方法によるものではありません。使用目的により、正確な含量が必要な場合は、それらの方法によって測定する必要があります。不活性ガス封入
-
説明
Indium lies in Group 13 (13th vertical column of the periodic table). it shows a wide variety of properties. It is considered to be metal of the 'poor metals' group.

Indium (symbol Ga; CAS# 7440-74-6) is widely used in the electronics industry, its radioisotopes are used for diagnostic imaging in medicine. Though this element is not essential for human nutrition, it is widely distributed in low concentrations in the environment (Smith et al., 1978).
Radioisotopes of indium have been used to label phagocytes and lymphocytes to localize inflammatory lesions (Dudley and Marrer, 1952; Abrams and Murrer, 1993). Despite early optimism, neither element has found wide use as a treatment for malignancies.
-
化学的特性
Indium is a rare, lustrous silver-white metal with atomic
number 49 and In as its atomic symbol. It is widely distributed
in the Earth’s crust in minute quantities (0.1 ppm) and is also
found in the hydrosphere. Indium belongs to group IIIA in the
periodic table. It was found and spectroscopically identified as
a minor component in zinc ores and isolated in 1863 by
Ferdinand Reich and Theodore Richter. Indium is so named
for the indigo blue color that its salts lend to flames. Indium
resembles tin in its physical and chemical properties and to
some extent in its toxicological properties. It is extremely soft
and malleable, with a Brinell test hardness of less than 1 and a
Mohs scale hardness of 1.2. In the electromotive series, it
appears between iron and tin and does not decompose inwater
at boiling temperature. It is stable in air, but when heated, it
burns with a nonluminous, blue-red flame yielding indium
oxide. The surface of indium remains bright up to its melting
point; above this, it forms an oxide film.
Indium is flammable in the form of dust, yielding indium
oxide when exposed to heat or flame. Mixtures of indium
with sulfur ignite when heated. Indium reacts explosively
with dinitrogen tetroxide plus acetonitrile. Indium exhibits
a violent reaction with mercury(II) bromide at 350°C. Indium
is unaffected by water, is attacked by mineral acids, and is
very resistant to alkalies.
-
物理的性質
Indium is silvery-white and malleable and looks much like aluminum and tin. However,it is softer than lead. Indium metal is so soft that it cannot be “wiped” onto other surfaces aswith a graphite pencil. Because it is noncorrosive and does not oxidize at room temperatures,it can be polished and will hold its shine better than silver. Its melting point is 156.60°C, itsboiling point is 2,075°C, and its density is 7.31 g/cm3.
-
同位体
There are a total of 73 isotopes of indium. All are radioactive with relativelyshort half-lives, except two that are considered stable. Isotope In-113 makes up just4.29% of the total indium found in the Earth’s crust. The isotope In-115, with a half-lifeof 4.41×10-14 years contributes the balance (95.71%) of the element’s existence in theEarth’s crust.
-
名前の由来
Indium’s name is derived from the Latin word indicum, meaning “indigo,”
which is the color of its spectral line when viewed by a spectroscope.
-
天然物の起源
Indium is a rather rare metal. It is the 69th most abundant element, which is about asabundant as silver at 0.05 ppm. Although it is widely spread over the Earth’s crust, it is foundin very small concentrations and always combined with other metal ores. It is never found inits natural metallic state.
Indium is recovered as a by-product of smelting other metal ores such as aluminum,antimony, cadmium, arsenic, and zinc. About 1,000 kg of indium is recovered each year (ora concentration of 1 part indium per 1000 parts of dust) from the flue stacks (chimneys) ofzinc refineries.
Indium is found in metal ores and minerals located in Russia, Japan, Europe, Peru, andCanada, as well as in the western part of the United States.
-
特性
Indium has one odd characteristic in that in the form of a sheet, like the metal tin, it willemit a shrieking sound when bent rapidly. Indium has some of the characteristics of othermetals near it in the periodic table and may be thought of as an “extension” of the secondseries of the transition elements. Although it is corrosion-resistant at room temperature, it willoxidize at higher temperatures. It is soluble in acids, but not in alkalis or hot water.
-
使用
Indium element is used in the synthesis of therapeutic particles containing metal ions; characterized by the use of unique ligand sets capable of making the metal ion complex soluble in biolgical medi
a to induce selective toxicity in diseased cells.
-
定義
indium: Symbol In. A soft silvery elementbelonging to group 13 (formerlyIIIB) of the periodic table; a.n.49; r.a.m. 114.82; r.d. 7.31 (20°C);m.p. 156.6°C; b.p. 2080±2°C. It occursin zinc blende and some iron oresand is obtained from zinc flue dust intotal quantities of about 40 tonnesper annum. Naturally occurring indiumconsists of 4.23% indium–113(stable) and 95.77% indium–115 (halflife6 × 1014 years). There are a furtherfive short-lived radioisotopes.The uses of the metal are small –some special-purpose electroplatesand some special fusible alloys. Severalsemiconductor compounds areused, such as InAs, InP, and InSb.With only three electrons in its valencyshell, indium is an electron acceptorand is used to dope puregermanium and silicon; it forms stableindium(I), indium(II), and indium(III) compounds. The elementwas discovered in 1863 by FerdinandReich (1799–1882) and HieronymusRichter (1824–90).
-
調製方法
Mineral sources are most commonly dark sphalerite (ZnS),
marmatite, and christophite (FeS:ZnS). Indium also occurs in small quantities in tin ores, siderite, and manganese and
tungsten ores.Gallium is often associated with indium in zinc
and tin ores. Many sulfide ores of copper, iron, lead, cobalt,
and bismuth contain small quantities of indium. Zinc smelter
flue dusts, in some cases, contain more than 1% indium, and
are the largest commercial source of the metal. Other commercial
sources are plant residues and dross from the refining
of zinc, lead, and cadmium. Indium is recovered from zinc
processing residues by acid leaching followed by chemical
separation from the accompanying elemental impurities such
as zinc, cadmium, aluminum, arsenic, and antimony. Final
purification by aqueous electrolysis of the salts at a controlled
potential yields a product of 99.9% purity. Canada and Peru
supply the greatest amounts of unwrought waste and scrap.
Next in order are Japan, Germany, and the United Kingdom.
The pattern of indium usage, and potential industrial hazard,
is 30% in solders, low-melting alloys, and coatings; 30% in
instrument applications and holding devices; 18% in electronic
components; 6% in nuclear reactor controls; and 16%
in research and other uses.
-
一般的な説明
Soft, ductile, shiny, silver-white metal. Mp: 155.6°C; bp: 2080°C. Density 7.31 g cm-3.
-
反応プロフィール
Indium is a non-combustible solid in bulk form but is flammable in the form of a dust. Reacts with strong oxidizing agents. Reacts explosively with dinitrogen tetraoxide dissolved in acetonitrile. Reacts violently with mercury(II)bromide at 350°C. Mixtures with sulfur ignite when heated.
-
危険性
Indium metal dust, particles, and vapors are toxic if ingested or inhaled, as are most of thecompounds of indium. This requires the semiconductor and electronics industries that useindium compounds to provide protection for their workers.
-
健康ハザード
Indium (In) and compounds
cause injury to the lungs, liver and kidneys in
animals.
There are no reports of toxicity in humans.
When indium was applied to the skin there was
no evidence of irritation.
-
使用用途
インジウムの主な用途に、液晶ディスプレイやタッチパネルがあります。構造上、液晶パネルには透明な電極が必要ですが、この透明な電極には酸化インジウムスズが利用されています。酸化インジウムスズは、インジウムの酸化物である(In2O3) に (SnO2) を添加した物質で、通称ITOと呼ばれています。
酸化インジウムスズ (ITO) で形成された薄膜 (ITO膜) は、可視光の透過性と導電性を併せ持っており、液晶パネルにおける透明な電極としての役割を果たしため多く用いられてきました。そのほか、インジウムをシリコン、ゲルマニウムにドーピングすることでp型半導体とすることができます。
また、インジウムは、常温でも柔らかく、展延性に優れていますので、や金属との接合性が非常に高いです。そのため、低温環境下でも使用可能なとして利用されたり、低融点合金のはんだとしても有用です。
-
工業用途
Indium (symbol In) is a silvery-white metal with a bluish hue, whiter than tin.It is very ductile and does not work-harden, because its recrystallization point is below normal room temperature, and it softens during rolling. The metal is not easily oxidized, but above its melting point, 157 C, it oxidizes and burns with a violet flame.
Indium is now obtained as a by-product from a variety of ores. Because of its bright color, light reflectance, and corrosion resistance, it is valued as a plating metal, especially for reflectors. It is softer than lead, but a hard surface is obtained by heating the plated part to diffuse the indium into the base metal. It has high adhesion to other metals. When added to chromium plating baths it reduces brittleness of the chromium.
The three largest uses of indium are in semiconductordevices, bearings, and low meltingpointalloys.
-
発がん性
Indium has not been tested for its
ability to cause cancer in animals. However, the probable
carcinogenic properties of indium are linked to alterations in
the synthesis and maintenance of enzyme systems that
metabolize organic carcinogens. A compromise in the ability
of these metabolic systems would lead to altered cellular
responses to organic carcinogenic substances.
-
純化方法
Before use, the metal surface is cleaned with dilute HNO3, followed by thorough washing with water and an alcohol rinse. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 856 1963.]
-
Structure and conformation
The space lattice of Indium belongs to the tetragonal system and its deformed face centered cubic
D4h (4 atoms within a unit cell) has lattice constants of a=0.4588 nm, c=0.4938 nm, and
In–In=0.324 nm.