Chlorine trifluoride
化学名:Chlorine trifluoride
CAS番号.7990-91-2
英語名:Chlorine trifluoride
CBNumberCB92130379
MFClF3
MW92.45
MOL File7990-91-2.mol
Chlorine trifluoride 化学特性,用途語,生産方法
-
説明
Chlorine trifluoride (CIF3) is a toxic, corrosive, very reactive liquefied compressed gas packaged in cylinders as a liquid under its own vapor pressure of 1.55 kg/cm2 at 21°C (22 psia at 70°F). CIF3 is a very useful chemical in operations requiring a highenergy fluorinating agent or incendiary material, especially since it can be handled at room temperatures.
Chlorine trifluoride is primarily of interest as a component in rocket fuels, in industrial cleaning and etching operations primarily in the semiconductor industry, nuclear reactor fuel processing and other industrial operations. -
化学的特性
Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. Boils at 53°F. It reacts with water to form chlorine and hydrofluoric acid with release of heat.
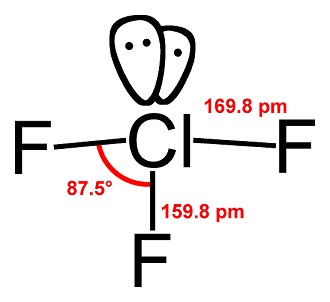
-
使用
Chlorine trifluoride is used as a fluorinating agent. It may be Used as a fluorinating agent, incendiary, igniter and propellant for rockets, in nuclear reactor fuel processing, pyrolysis inhibitor for fluoro carbon polymers.
During Word War II, Chlorine trifluoride was used by Germany as an incendiary gas. -
製造方法
Chlorine trifluoride was first reported by Ruff and Krug who prepared it by fluorination of chlorine, this also produced ClF and the mixture was separated by distillation.
3F2 + Cl2 → 2ClF3 -
健康ハザード
Chlorine trifluoride is toxic by itself and also reacts with moisture to form a variety of other toxic and corrosive materials, including hydrofluoric acid. When the product escapes into the environment, it hydrolyzes with the moisture in the air or, in the case of human contact, with the moisture in the human body. Direct contact with CIF3 vapor or liquid can result in a thermal burn in addition to the chemical burns produced by the hydrolysis products. -
化学反応性
Chlorine trifluoride is hypergolic (will initiate the combustion of many materials without an ignition source) with many materials. It is extremely reactive with most inorganic and organic materials. These reactions can be very violent or in some cases explosive. Therefore, all materials that come into contact with chlorine trifluoride must be evaluated.
Chlorine trifluoride hydrolyzes rapidly with moisture to form mostly hydrogen fluoride along with hydrogen chloride, chlorine monofluoride, and a variety of oxyhalogen compounds. The oxyhalogens may include chlorine dioxide, chlorous acid, chlorine oxyfluoride and oxygen difluoride.
Chlorine trifluoride is a strong oxidizer that can essentially decrease the ignition temperature of potential fuels, including materials of construction (e.g., metals) for CIF3 systems. Furthermore, because of chlorine trifluoride’s extreme reactivity, there is a high potential for contamination to serve as an ignition source. Friction between two materials can generate fine particles (contaminants), which may ignite from the heat generated. Contaminants in chlorine trifluoride systems potentially can burn with sufficient heat to propagate the ignition to system components. -
廃棄物の処理
Return unused product to the supplier for proper disposal. In process applications, gaseous chlorine trifluoride can be disposed of in either liquid or dry scrubbers. Dry scrubbers work well under normal operating conditions for small quantities of Chlorine trifluoride but are not recommended for large or emergency releases unless specifically designed. Scrubbers must be designed to withstand the heat generated in the event of a large release. For normal operation, it is recommended an inert gas be used as a diluents prior to product being introduced to scrubber. This will help disperse the heat of reaction. Wet scrubbers typically use caustic solutions, such as potassium or sodium hydroxide, as the scrubbing medium. Wet scrubbers handled the heat of reaction better, as well as neutralize the products of reaction. Disposal of liquid chlorine trifluoride is extremely hazardous and is not recommended.
Chlorine trifluoride 生産企業
Global(13)Suppliers
-
Shanghai Daken Advanced Materials Co.,Ltd
電話番号 +86-371-66670886
電子メール info@dakenam.com
-
Shaanxi Dideu Medichem Co. Ltd
電話番号 +86-029-89586680<br/>+86-18192503167
電子メール 1026@dideu.com
-
電話番号 +8615986615575
電子メール info@codchem.com
-
Henan Fengda Chemical Co., Ltd
電話番号 +86-371-86557731<br/>+86-13613820652
電子メール info@fdachem.com
-
SHANGHAI KEAN TECHNOLOGY CO., LTD.
電話番号 +8613817748580
電子メール cooperation@kean-chem.com
-
HUNAN CHEMFISH SCIENTIFIC CO.,LTD
電話番号 +86-0731-85567275<br/>18932438858
電子メール sales05@chemfish.com
-
Isotope (Xiamen) Industry and Trade Co., Ltd
電話番号 0510-051082803581<br/>15903302207
電子メール zhongxin326@sohu.com
-
電話番号 131-9467-7939<br/>4001882517
電子メール shineliu@shanglangas.com
-
Shandong Hemao New Material Co., Ltd.
電話番号 13061243681
電子メール hemiao66@163.com
-
電話番号 0731-85567275<br/>18229978283
電子メール tokyo@chemfish.com
1of2