-
外観
無色~わずかにうすい黄色, 澄明の液体
-
性質
イソプレンの化学式C5H8です、 英語名Isopreneです、分子量68.12です、融点は−145.95℃で、沸点は34.067℃であり、室温では揮発性が高い無色の液体です。ゴムや都市ガスのような臭気を有します。可燃性や引火性に富んでおり、大気中に霧状で存在する場合には爆発の危険性があります。
-
溶解性
水に微溶 (440mg/L), アルコール, エーテルに易溶。エタノール及びアセトンに極めて溶けやすく、水にほとんど溶けない。
-
解説
イソプレンはい脂肪族不飽和炭化水素(ジエン)の一つ.分枝メチル基と共役二重結合とをもっている.天然ゴムの構成単位であり,石油系炭化水素の分解ガス中にも含まれる.
構造はブタジエンの内部炭素に結合している水素の一つをメチル基で置換した構造をもつ.無色のやや刺激臭のある揮発性の液体.融点-145.95 ℃,沸点34.07 ℃.d2540.67587.n25D1.41852.各種有機溶剤に可溶,水に不溶.[CAS 78-79-5]
森北出版「化学辞典(第2版)
-
用途
イソプレンゴム、ブチルゴム等のゴム類合成のための中間原料
-
構造
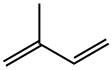
イソプレンは二重結合を2個有するジエンの1種です。化学式はC5H8で表され、分子量は68.12です。2-メチル-1,3- (英: 2-methyl-1,3-butadiene) とも呼ばれます。
-
使用する
共役オレフィンとしてのイソプレン,石油化学工業における重要な炭化水素中間体の一つ.おもな用途はイソプレンゴム(cis-1,4-ポリイソプレン),ブチルゴムなどの合成ゴム原料,およびファインケミカル用合成原料である.
-
準備
製法は,天然ゴム,テレビン油などを熱分解して得られる.工業的には,石油系炭化水素の熱分解により,エテンを製造する際に副生する C5 留分から,抽出蒸留により分離する方法,イソペンテンを脱水素する方法,イソブテンとホルムアルデヒドを脱水縮合する方法などがある.
-
合成
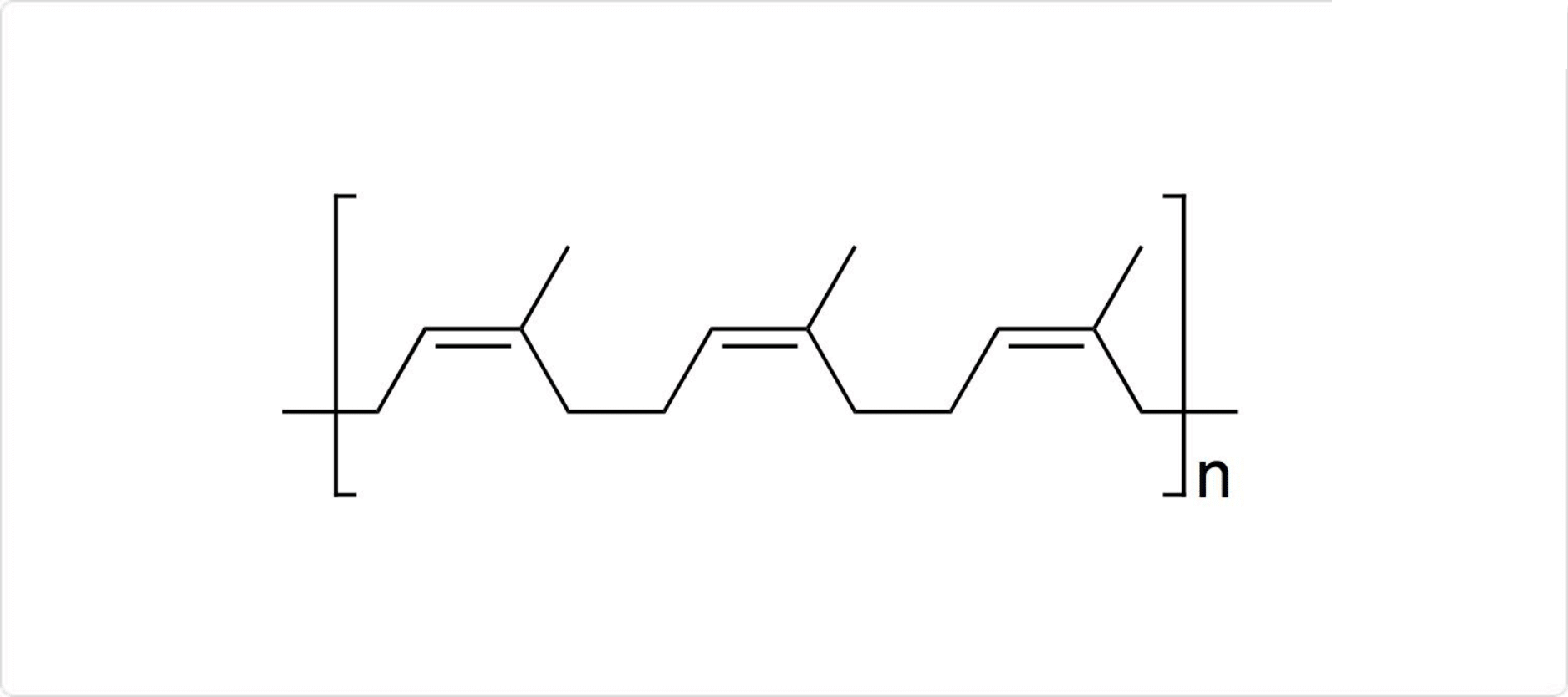
図1. ポリイソプレンの構造
天然ゴムを熱分解することで、初めてイソプレンが単離されました。工業的にイソプレンは、毎年800,000トンほど製造されます。生産されたイソプレンの95%は、人工天然ゴムであるシス-1,4-ポリイソプレンの合成のためのモノマーとして使用されます。
天然ゴムは、10万~100万個のイソプレン分子から構成された付加重合体です。基本構造はほとんどシス-1,4-ポリイソプレンです。ただし、天然ゴム中には、シス-1,4-ポリイソプレンの立体異性体であるトランス-1,4-ポリイソプレンが、わずかに含まれている場合もあります。それ以外にも天然ゴムには、脂肪酸、たんぱく質、無機物などが微量に含まれています。
-
使用上の注意
アルゴン封入
-
化学的特性
Isoprene (2-methyl-l,3-butadiene) is a colorless, volatile, flammable liquid with specific gravity 0.6758. It is highly reactive, usually occurs as its dimer, and unless inhibited undergoes explosive polymerization. Isoprene naturally occurs in the environment as emissions from vegetation. It may be released to the environment as emissions during wood pulping, biomass combustion, and rubber abrasion; through tobacco smoke, gasoline, turbine, and automobile exhaust. In tobacco smoke, isoprene has been determined to be the precursor of a number of polycyclic aromatics, as demonstrated by thermal condensations in the range of 450–700℃.
-
物理的性質
Colorless, volatile, extremely flammable liquid with an petroleum-like odor. An odor threshold concentration of 48 ppbV was reported by Nagata and Takeuchi (1990).
-
使用
Isoprene occurs in nature and it is produced by many plants. Its polymers are the main component of natural rubber. The most important application of isoprene is to manufacture polymers and copolymers. Polyisoprene, a synthetic rubber made from isoprene, is used in a wide variety of rubber applications including medical equipment, baby bottle teats/nipples, toys, shoe soles, tires, elastic films, threads for golf balls or textiles, adhesives, paints, and coatings. Copolymer butyl rubber, made from isobutene with a small amount of isoprene, has excellent impermeability to gases and is used in inner tubes. Another copolymer styrene-isoprene rubber is used in pressure sensitive adhesives. Isoprene is also used as a chemical intermediate.
-
製造方法
Isoprene is obtained from propylene by the followin,g route:
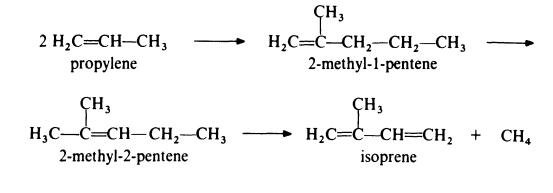
In the first step, propylene is dimerized to 2-methyl-l-pentene by passage
over a catalyst of tri-n-propylaluminium at about 200??C and 20 MPa (200
atmospheres). This product is then isomerized to 2-methyl-2-pentene by
heating at 150-300??C in the presence of a silica-alumina catalyst. The final
step in the process is the pyrolysis of the olefin to isoprene at 650-800??C in
the presence of a free radical initiator such as hydrogen bromide. The
isomerization step is necessary because pyrolysis of 2-methyl-l-pentene gives
much poorer yields of isoprene than pyrolysis of 2-methyl-2-pentene.
-
定義
ChEBI: A hemiterpene with the formula CH22C(CH3)CH2CH2; the monomer of natural rubber and a common structure motif to the isoprenoids, a large class of other naturally occurr
ng compounds.
-
調製方法
Rubber results from the polymerization of isoprene to form polyisoprene. The resultingstructure dictates the properties of the rubber. Natural rubber has a cis 1,4 structure.This means that the carbon atoms that form the chainattach to the same side ofthe chain at the 1 and 4 positions. The cisstructure gives rubber its elasticity. Polyisoprene alsoexists in a trans 1,3 configuration. In the trans configuration, the addition takes place onopposite sides of the carbon chain.
Natural rubber occurs in a colloidal milky suspension called latex, which is obtained fromnumerous plants. The most important of these is the para rubber tree, Hevea brasiliensis. Naturalrubber is harvested by cutting a v-shape incision into a plant and allowing latex to drain intoa container containing a preservative. About 50mL of latex is obtained on a daily basis. Latexis transported to collection stations where it is processed for shipment. Processing can includepreservation, coagulation, and concentrating before being sent to rubber factories.
-
一般的な説明
A clear colorless liquid with a petroleum-like odor. Density 5.7 lb / gal. Flash point -65°F. Boiling point 93°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a closed container, the container may rupture violently. Less dense than water and insoluble in water. Vapors heavier than air.
-
空気と水の反応
Highly flammable. Insoluble in water.
-
反応プロフィール
ISOPRENE may react vigorously with strong oxidizing agents. May react exothemically with reducing agents to release hydrogen gas. May undergo exothermic addition polymerization in the presence of various catalysts (such as acids) or initiators. Undergoes autoxidation upon exposure to the air to form explosive peroxides. Mixing isoprene in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid (70%), oleum, sulfuric acid (90%) [NFPA 1991].
-
危険性
Highly flammable, dangerous fire and
explosion risk. Irritant. Possible carcinogen.
-
健康ハザード
Vapor produces no effects other than slight irritation of the eyes and upper respiratory tract. Liquid may irritate eyes; like gasoline.
-
類似物
1. イソプレンを構造単位に持つ天然化合物
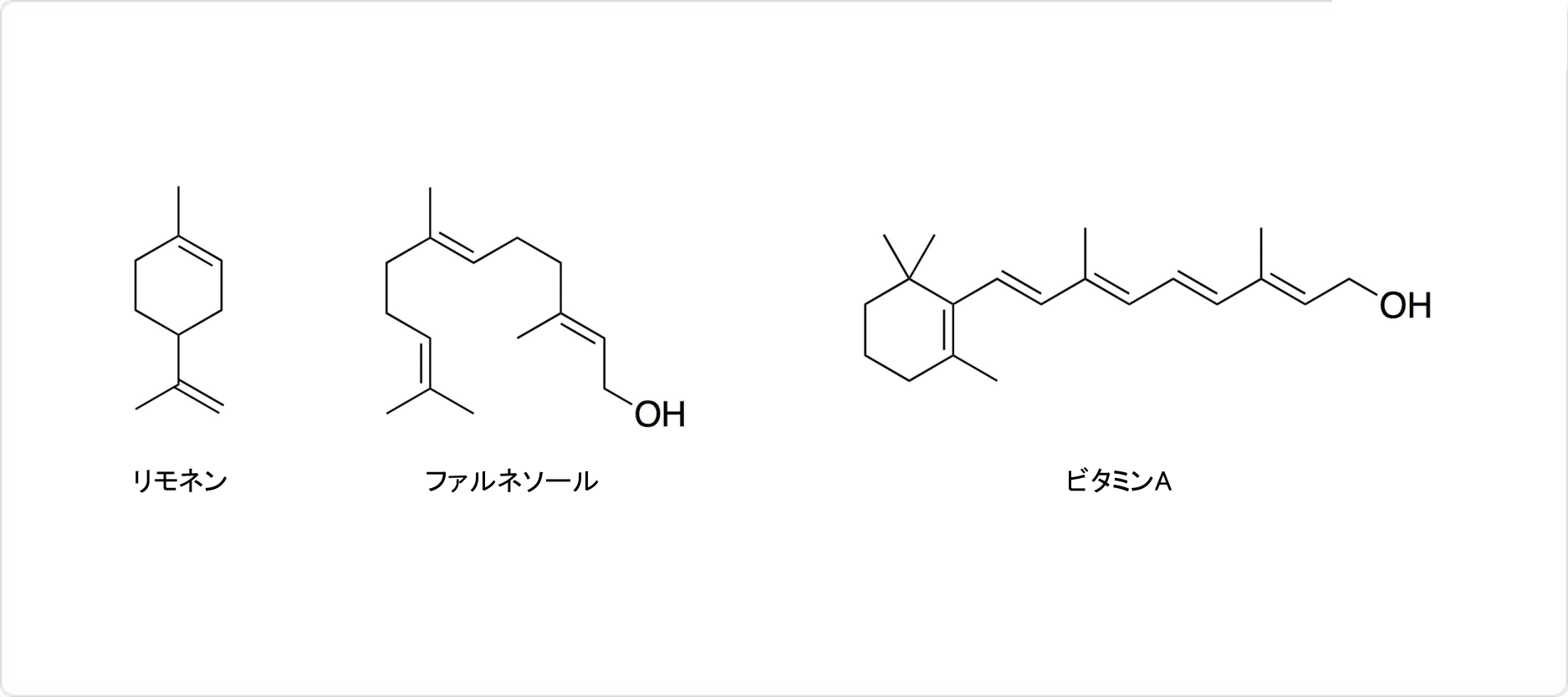
図2. テルペンの構造
イソプレノイド (英: isoprenoid) やテルペノイド (英: terpenoid) と呼ばれる天然有機化合物は、イソプレンを構成単位としています。生体物質として、昆虫、植物、細菌、菌類が作っており、精油の中から発見された10個の炭素の有する化合物に与えられた名称です。これらの炭化水素の分子式は(C5H8)nであり、イソプレンの倍数で表されます。
イソプレノイドやテルペノイドの具体例として、2個のイソプレン単位を持つリモネンや、3個のイソプレン単位を持つファルネソールなどが挙げられます。リモネンやファルネソールは、香料として使用可能です。4つのイソプレン単位から構成されるビタミンAもテルペノイドです。
2. 天然化合物中の機能性イソプレンユニット
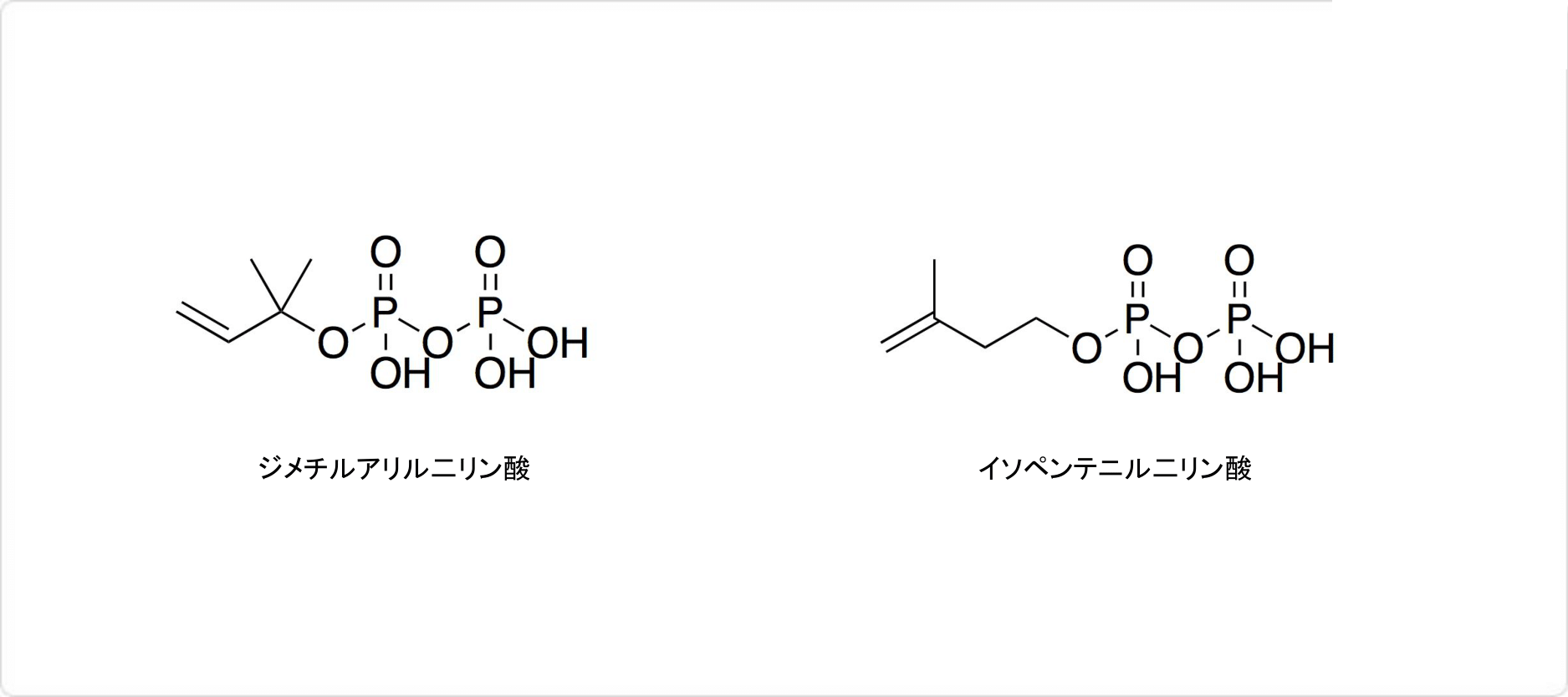
図3. 天然化合物中のイソプレンユニット
テルペンやテルペノイドの生合成に必要となる前駆物質にも、イソプレン単位が含まれています。生物システムにおいて、機能を有するイソプレン単位は、ジメチルアリル二リン酸 (英: dimethylallyl pyrophosphate) とイソペンテニル二リン酸 (英: isopentenyl diphosphate) です。イソペンテニル二リン酸は、ジメチルアリル二リン酸の異性体です。
参考文献
-
化学性质
天然ゴムの構成要素であることから,合成ゴムの原料として利用される。
-
使用用途
主にイソプレンは、合成ゴムであるポリイソプレンゴムやの原料として用いられています。ポリイソプレンゴムは、その約70%が自動車や航空機のタイヤに使用されます。
加えて、生体適合性が高いため、医療分野でも使用可能です。具体的には、超音波診断機用のゴム袋や血液回路の部品などに使われます。
イソプレンの合成ゴムの原料以外の用途として、ゲラニオール、リナロール等の原料、香料原料、菊酸等の農薬中間体原料などが挙げられます。
-
発がん性
Isoprene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
-
環境運命予測
At 25 ℃, isoprene has a high vapor pressure of 733 hPa, a low
water solubility of 642 mg l-1, and a Henry’s law constant of
7781 Pam3 mol-1. Isoprene’s log Kow is 2.42 while its log Koc
is 1.83. Isoprene’s vapor density relative to air is 2.4. Because of
its high vapor pressure at ambient temperature, isoprene will
partition largely into the atmosphere, with negligible amounts
partitioning to soil and water. Due to a short half-life in air
(0.5 h by reaction with nitric oxide, 1.2–4 h by reaction with
hydroxyl radicals, and 19 h by reaction with ozone), wet
deposition of isoprene from air is not expected to play
a significant role in its atmospheric fate. Although laboratory
testing demonstrates that isoprene has the potential to biodegrade,
microbial metabolism is unlikely to contribute significantly
to the removal of isoprene from the environment due to
rapid volatilization from terrestrial and aquatic media.
Isoprene has a low bioaccumulation potential and is not
expected to bioaccumulate.
-
合成方法
抽出法と合成法に分けられる。抽出法は,ナフサ分解のC5留分からジメチルホルムアミドなどの溶剤を用いて抽出する方法である。合成法には,イソペンタン,イソペンテンを脱水素する方法と,イソブチレンにホルムアルデヒドを付加(Prince反応)することで得られる4,4-ジメチル-1,3-ジオキサンを分解する方法がある。
-
純化方法
Reflux it with sodium then distil it from sodium or NaBH4 under nitrogen, and pass it through a column containing KOH, CaSO4 and silica gel. tert-Butylcatechol (0.02% w/w) is added, and the isoprene is stored in this way until redistilled before use. The inhibitor (tert-butylcatechol) in isoprene can be removed by several washings with dilute NaOH and water. The isoprene is then dried over CaH2, distilled under nitrogen at atmospheric pressure, and the fraction distilling at 32o is collected. Store it under nitrogen at -15o. [Beilstein 1 H 252, 1 IV 1001.]