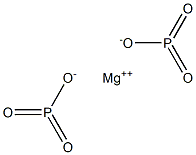
次りん酸マグネシウム
化学名:次りん酸マグネシウム
英語名:Magnesium hypophosphate
CBNumberCB42345956
MFMgO6P2
MW182.248922
MOL FileMol file
别名
次りん酸マグネシウム
more