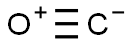-
性質
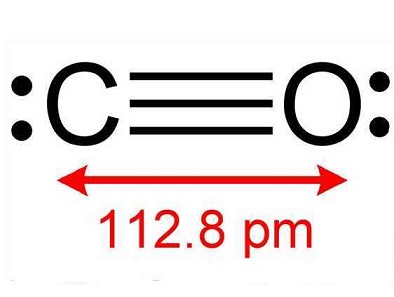
無色無味の有毒気体で、無臭であるだけにたいへん危険である。高温では還元剤として働き、多くの金属酸化物を還元して金属を生成する。水に溶けにくい。常温、常圧では水酸化ナトリウム水溶液と反応しないが、高温高圧下では反応してギ酸塩となる。空気中で点火すると青白い炎をあげて燃え二酸化炭素となる。ハロゲン、硫黄(いおう)など非金属元素と結合する。また、高温で遷移金属元素と金属カルボニル(ニッケルカルボニルなど)とよばれる化合物をつくる。適当な温度、圧力、触媒などでいろいろな合成反応の原料となる。たとえば、水素との直接結合によりメタノール(メチルアルコール)、アルケンに対し水素とともに反応してアルデヒドを生じる。一酸化炭素の物理的性質は窒素ガスにたいへんよく似ている(原子価電子の総数が同じであることに注意)。一酸化炭素のC-O結合距離は113ピコメートルで、アルデヒドやケトンなどのカルボニル化合物のC-O距離122ピコメートル(二重結合)より短く、三重結合に近いものと考えられている。
-
解説
化学式はCO。融点−205℃,沸点−191.6℃。無色,無臭,水に微溶の気体。有毒。空気中では青い炎をあげて燃え,強い還元性がある。炭素または炭素化合物が不完全燃焼するとき生ずる。発生炉ガス,水性ガスなどの主成分で,天然ガス以外の家庭用都市ガスにも含まれている。燃料および鉱石の還元に多く使われ,メタノールの製造をはじめ有機合成化学工業の原料としても重要。→関連項目環境基準|工業中毒|自動車排出ガス規制
株式会社平凡社 百科事典マイペディアについて 情報
-
用途
金属カーボニル、メタノール、アンモニア原料。冶金(ニッケルのモンド法)、有機合成(フィッシャー?トロプシュ法)に使用される。多くの工業的用途では窒素や水素との混合物のままで使用される
-
製造
工業的には、コークスまたは石炭を原料としてつくる。熱したコークスに空気を通すと、一酸化炭素と窒素の混合物(発生炉ガス)が得られ、また、水蒸気を通すと、一酸化炭素と水素との混合気体(水性ガス)が得られる。いずれも気体燃料である。
2C+O2―→2CO(発生炉ガス法)
C+H2O―→CO+H2(水性ガス法)
実験室では、ギ酸を濃硫酸中に滴下して脱水してつくるが、純粋なものはニッケルカルボニルNi(CO)4を200℃で分解して得られる。
水またはアルカリ水溶液で洗い、必要な場合は液化させてから分留する。また、塩化銅(Ⅰ)のアンモニアアルカリ性溶液に吸収させてから、再発生させる方法もある。[守永健一・中原勝儼]
-
用途
発生炉ガス、水性ガスなどの主成分として工業用気体燃料、また金属の冶金(やきん)に還元剤として広く用いられる(溶鉱炉中の酸化鉄の還元Fe2O3+3CO―→2Fe+3CO2など)。一酸化炭素そのものやホスゲンCOCl2をつくり、これらを出発物質として各種化合物を合成することも重要な用途の一つとなっている。
-
注意
一酸化炭素は,毒性が強く、空気中での許容濃度は50ppmとされる。血液中のヘモグロビンと結合し、その作用を阻害するためである。また引火性が強く、空気と混合すると、きわめて爆発しやすい。
-
効能
免疫調節薬
-
説明
Carbon monoxide is a colorless, odorless, tasteless, flammable, toxic gas.Carbon monoxide is produced when carbon and carbon compounds undergo incomplete combustion. The inefficient combustion of carbon fuels for heating results in the production of carbon monoxide, which may result in high CO concentrations in indoor environments. The use of carbon fuel heaters without adequate ventilation can result in deadly conditions. Each year several hundred people in the United States die from CO poisoning, and 10,000 patients are treated in hospitals for CO exposure.Cars and other forms of transportation are a major source of carbon monoxide pollution in cities.
-
化学的特性
Carbon monoxide, CO, is a colorless,odorless, toxic gas. It is soluble in alcohol and cuprix chloride solutions, but insoluble in water. Carbon monoxide is formed by the incomplete oxidation of carbon. It is found in mines and carexhaust. Carbon monoxide is used in metallurgy as a reducing agent in smelting operations, in the production of carbony is for the separation of various metals, as an ingredient in the synthesis of phosgene,and as an intermediate in the production of methanol.
Because it is only slightly less dense than air, it mixes readily without stratification. Because it is only slightly less dense than air, it mixes readily without stratification. Because of its low boiling point, carbon monoxide is shipped as a nonliquified compressed gas. It is also known as carbon oxide, flue gas, and monoxide. Carbon monoxide is a flammable gas and is incompatible or reactive with strong oxidizers, such as bromine trifluoride, chlorine trifluoride, and lithium.
-
物理的性質
Colorless, odorless and tasteless gas; density 1.229 g/L; very flammable,burns in air with a bright blue flame; liquefies at -191.5°C; solidifies at -205°C; critical temperature -140°C, critical pressure 34.53 atm, critical vol ume 93 cm3/mol; soluble in chloroform, acetic acid, ethyl acetate, ethanol, and ammonium hydroxide; sparingly soluble in water (2.3 mL/100 mL water at 20°C).
-
天然物の起源
Carbon monoxide is found in varying concentrations in unventilated and confined spaces resulting from partial oxidation of carbonaceous matter. Burning wood, paper, kerosene, or other organic materials in inadequate air can produce this gas. It also is found in automobile exhaust and tobacco smoke emissions.
Carbon monoxide has many important industrial applications. It is used in Fischer-Tropsch process to produce liquid or gaseous hydrocarbons, synthet ic fuels and many oxygenated derivatives. This process was applied before and during World War II to produce synthetic fuels. Probably the most important application of this compound involves production of oxygenated organics in the Synthol process and in oxo synthesis. Many aliphatic alcohols, alehydes and ketones are produced by catalytic hydrogenation of carbon monoxide. Oxo synthesis produces aldehydes from olefins. Carbon monoxide also is the start ing material for preparing metal carbonyls. In metallurgy, it is used as a reducing agent to reduce oxides. In the Mond process it recovers nickel.
-
来歴
Carbon monoxide is a colorless, odorless, tasteless, flammable, toxic gas. It was first identified by the Spanish alchemist Arnold of Villanova (1235–1313), who noted the production of a poisonous gas when wood was burned. The formal discovery of carbon monoxide is credited to the French chemist Joseph Marie Fran?ois de Lassone (1717–1788) and the British chemist Joseph Priestley (1733–1804). The former prepared carbon monoxide by heating carbon in the presence of zinc, and for a time the compound was incorrectly identified as hydrogen. William Cumberland Cruikshank (1745–1800) correctly determined that carbon monoxide was an oxide of carbon in 1800.
-
使用
Carbon monoxide is used in the oxo processor Fischer–Tropsch process in the produc tion of synthetic fuel gas (producer gas, watergas, etc.); as a reducing agent in the Monodprocess for the recovery of nickel; in car bonylation reactions; and in the productionof metal carbonyls and complexes. It is pro duced by incomplete combustion of organicmaterials. Risk of exposure to this gas arisesunder fire conditions; from a burning stoveor from burning wood or candles in a closedroom; in the exhausts of internal combustion engines; in a closed garage with theautoengine on; and from oil or gas burners,improperly adjusted.
-
定義
A colorless flammable toxic gas
formed by the incomplete combustion of
carbon. In the laboratory it can be made by
dehydrating methanoic acid with concentrated
sulfuric acid:
HCOOH – H2O → CO
Industrially, it is produced by the oxidation
of carbon or of natural gas, or by the
water-gas reaction. It is a powerful reducing
agent and is used in metallurgy.
Carbon monoxide is neutral and only
sparingly soluble in water. It is not the anhydride
of methanoic acid, although under
extreme conditions it can react with
sodium hydroxide to form sodium
methanoate. It forms metal carbonyls with
transition metals, and its toxicity is due to
its ability to form a complex with hemoglobin.
-
調製方法
Carbon monoxide is formed during combustion of carbonaceous
materials in oxygen (when carbon is in excess), or it
can be formed (with oxygen) by thermal decomposition
of carbon dioxide (>2000° °C). It can be generated by improperly vented cooking and heating appliances including
coal stoves, furnaces, and gas appliances when the oxygen
supply is insufficient. Other sources include exhaust of
internal combustion engines, structural fires, and tobacco
products. Carbon monoxide can also be formed endogenously
by normal heme turnover or during the metabolism
of selected hydrocarbons, such as methylene chloride. Not
surprisingly, CO is one of the most common agents of
inadvertent human intoxication in both occupational and
nonoccupational environments.
-
一般的な説明
A colorless cryogenic liquid. Prolonged exposure to carbon monoxide rich atmospheres may be fatal. Contact with the liquid can cause severe frostbite. Less dense than air. Easily ignited and a flame can flash back to the source of a leak very easily. Burns with a violet flame. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. CARBON MONOXIDE is used in organic synthesis, metallurgy, and a fuel.
-
空気と水の反応
Highly flammable.
-
反応プロフィール
Contact of very cold liquefied gas with water may result in vigorous or violent boiling and extremely rapid vaporization. If the water is hot, a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if the liquid contacts water in a closed container [Handling Chemicals Safely 1980]. Reacts explosively with bromine trifluoride at high temperatures or concentrations [Mellor 2, Supp. 1:166 1956]. The same is true for various oxidizers such as: chlorine dioxide, oxygen (liquid), peroxodisulfuryl difluoride. Reacts with lithium to give lithium carbonyl, which detonates violently with water, igniting the gaseous products [Mellor 2, Supp 2:84 1961]. Potassium and sodium metals behave similarly. Cesium oxide, iron(III) oxide, and silver oxide all react, in the presence of moisture, at ambient temperatures with carbon monoxide causing ignition, [Mellor, 1941, vol. 2, 487].
-
危険性
Highly flammable, dangerous fire and
explosion risk. Flammable limits in air 12–75% by
volume. Toxic by inhalation. Note: Carbon monox-
ide has an affinity for blood hemoglobin over 200
times that of oxygen. A major air pollutant.
-
火災危険
EXTREMELY FLAMMABLE. May be ignited by heat, sparks or flames. Flame may be invisible. Containers may explode when heated. Vapor explosion and poison hazard indoors, outdoors or in sewers. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Runoff may create fire or explosion hazard.
-
燃焼性と爆発性
Carbon monoxide is a flammable gas. It forms explosive mixtures with air in the
range of 12.5 to 74% by volume.
-
一酸化炭素中毒
一酸化炭素中毒とは、一酸化炭素が原因となる中毒症状のことです。頭痛、めまい、脱力感、嘔吐、胸痛、錯乱などの症状が現れます。一酸化炭素がヘモグロビンと結合して、酸素を運搬する機能を妨げることで、中毒症状を引き起こします。
1時間の暴露では、症状は500ppmで現れ始め、1,000ppmでは顕著な症状が現れ、1,500ppmで死亡に至ります。症状が風邪に似ているため、火事などが原因でない場合には、気づかず一酸化炭素を吸い続け、急速に昏睡に陥り、気付かずに死亡することも珍しくありません。
対策として、一酸化炭素ガスが発生する危険性のある箇所に、一定以上の濃度になるとアラームが鳴るガス警報器を使用することなどが挙げられます。
-
化学性质
色の有毒気体で,空気中で燃焼し,金属酸化物を還元する。
-
使用用途
一酸化炭素は、発生炉ガスや水性ガス等の主成分として、工業用気体燃料に利用される他に、金属の冶金を行う際に、還元剤としても広く用いられています。、アンモニア原料などをはじめとする、工業上重要な化合物を製造する際の原料としても、非常に重要な化合物です。
一酸化炭素は、特殊な条件下で触媒を作用させると、多くの遷移金属と反応し、金属カルボニルをつくります。例えば、ニッケルカルボニルやコバルトカルボニルは、レッペ反応やオキソ反応の触媒として、有機合成化学において重要な化合物です。
-
工業用途
Carbon monoxide (CO) is a product of incompletecombustion, and is very reactive. It is oneof the desirable products in synthesis gas formaking chemicals; the synthesis gas made fromcoal contains at least 37% CO. It is also recoveredfrom top-blown O2 furnaces in steel mills.It reacts with H2 to form methanol, which is then catalyzed by zeolites into gasoline. Aceticacid is made by methanol carbonylation, andacrylic acid results from the reaction of CO,acetylene, and methanol.
-
材料の用途
Steel and other common metals are satisfactory
for use with dry, sulfur-free carbon monoxide at
pressures up to 2000 psig (13 790 kPa). Iron,
nickel, and other metals can react with carbon
monoxide at elevated pressures to form carbonyls
in small quantities. The presence of moisture
and sulfur-containing impurities in carbon monoxide
appreciably increases its corrosive action
on steel at any pressure. High-pressure plant
equipment is often lined with copper for increased
resistance to carbon monoxide attack.
Very highly alloyed chrome steels are sufficiently
resistant to corrosion by carbon monoxide
containing small amounts of sulfur-bearing
impurities. Users are strongly urged to make
stress corrosion tests of samples of proposed
construction materials in order to select one that
will withstand the high-pressure use of carbon
monoxide under actual conditions.
-
職業ばく露
Carbon monoxide is used in metallurgy as a reducing agent, particularly in the Mond process
for nickel; in organic synthesis, especially in the
Fischer�Tropsch process for petroleum products, and in
the oxo reaction; and in the manufacture of metal carbonyls. It is usually encountered in industry as a waste product of incomplete combustion of carbonaceous material
(complete combustion produces CO2). The major source of
CO emission in the atmosphere is the gasoline-powered
internal combustion engine. Special industrial processes
which contribute significantly to CO emission are iron
foundries, particularly the cupola; fluid catalytic crackers;
fluid coking; and moving-bed catalytic crackers in thermal
operations in carbon black plants; beehive coke ovens;
basic oxygen furnaces, sintering of blast furnace feed in
steel mills; and formaldehyde manufacture. There are
numerous other operations in which a flame touches a surface that is cooler than the ignition temperature of the gaseous part of the flame where exposure to CO may occur,e.g., arc welding, automobile repair; traffic control; tunnel
construction; firefighting; mines, use of explosives, etc.
-
概要
一酸化炭素とは、炭素の酸化物の1種であり、常温常圧で無色無臭、可燃性の気体です。
酸素が不十分な環境下で、炭素や炭素化合物が燃焼したり、高温環境下でがコークスによって還元されたりする際に発生します。一酸化炭素は、ボンベ入りで市販されています。また、石炭や石油などを多量に消費する工場地帯の大気中には、多量の一酸化炭素が存在することもあります。
-
環境運命予測
CO has varied effects on multiple enzymatic reactions and
processes. Most easily seen and measured via co-oximetry is its
high affinity and binding to Hb. This results in an overall lack
of oxygen carrying capacity along with a shift of the oxygen
dissociation curve to the left so that even available oxyhemoglobin
is less able to offload oxygen to tissue sites. This,coupled with CO’s ability to bind to and arrest cellular
metabolism, results in global hypoxemia. The overall lack of
tissue perfusion and energy production results in metabolic
lactic acidosis.
CO also has the ability to bind to other globins, most
importantly myoglobin. Significant myoglobin binding
results in lack of tissue oxygenation to heart and myocardial
damage.
The final high-risk organ system affected after CO exposure
is the central nervous system. CO has the ability to cause
delayed neuropsychiatric sequelae in addition to the acute
effects seen as a result of hypoxemia. This is thought to be due
to delayed lipid peroxidation achieved through the displacement
of nitric oxide. A reperfusion-like injury occurs in these
cases.
-
貯蔵
cylinders of carbon monoxide
should be stored and used in a continuously ventilated gas cabinet or fume hood.
Local fire codes should be reviewed for limitations on quantity and storage
requirements.
-
輸送方法
UN1016 Carbon monoxide, compressed,
Hazard class: 2.3; Labels: 2.3-Poisonous gas; 2.1-
Flammable gas, Inhalation Hazard Zone D. NA9202
Carbon monoxide, refrigerated liquid (cryogenic liquid),
Hazard class: 2.3; Labels: 2.3-Poisonous gas; 2.1-
Flammable gas, Domestic (United States), Inhalation
Hazard Zone D. Cylinders must be transported in a secureupright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the
compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas
cylinders without the express written permission of the
owner.
-
合成方法
炭素の不完全燃焼あるいは二酸化炭素と炭素の高温反応で生成する。
-
純化方法
Iron carbonyl is a likely impurity in CO stored under pressure in steel tanks. It can be decomposed by passing the gas through a hot porcelain tube at 350-400o. Passage through alkaline pyrogallol solution removes oxygen (and CO2). Removal of CO2 and water are effected by passage through soda-lime followed by Mg(ClO4)2 or P2O5 and collected over Hg. Carbon monoxide can be condensed and distilled at -195o. It is sparingly soluble in H2O but is readily absorbed by a solution of CuCl in HCl to give the white crystalline adduct CuCl.CO.2H2O. It burns in air with a bright blue flame but a mixture of 2volumes of CO and 1volume of O2 explode when kindled, although in a small jar the combustion is not violent. HIGHLY POISONOUS gas as it reacts with haemoglobin to form bright red carboxyhaemoglobin which is stable and not readily decomposed by oxygen. [Gilliland & Blanchard Inorg Synth II 81 1946, Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 645-646 1963.]
-
不和合性
Forms extremely explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. In the presence of finely dispersed
metal powders the substance forms toxic and flammable
carbonyls. May react vigorously with oxygen, acetylene,
chlorine, fluorine, nitrous oxide.
-
廃棄物の処理
Return refillable compressed
gas cylinders to supplier. Dissolve or mix the material with
a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. Carbon monoxide can also be recovered from
gas mixtures as an alternative to disposal.
-
予防処置
Carbon monoxide poisoning, prevention, occupational safety
Install a CO alarm on each level of your home.
Home heating systems, chimneys, and fl ues must be inspected and cleaned by a qualifi ed technician every year. Keep chimneys clear of bird and squirrel nests, leaves, and residue to ensure proper ventilation.
Make sure that the furnace and other appliances, such as gas ovens, ranges, and cooktops are inspected for adequate ventilation.
Do not burn charcoal inside the house even in the fi replace.
Do not operate gasoline-powered engines in confi ned areas, such as garages or basements. Do not leave your car, mower, or other vehicle running in an attached garage, even with the door open.
Do not block or seal shut exhaust fl ues or ducts for appliances such as water heaters, ranges, and clothes dryers.