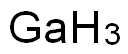-
外観
無色澄明の液体
-
性質
ガリウムは、融点が29.8℃、沸点が2,403℃であり、常温では固体または液体として存在し、固体状態での密度は5.91g/cm3です。金属のなかでは、融点が低いというのがガリウムの最も知られている特徴です。固体状態では反磁性ですが、液体状態では常磁性となり、40℃における磁化率は2.4×10−6です。
-
解説
周期表第13族に属する元素。1875年、フランスのボアボードランが分光分析によりピレネー産の閃(せん)亜鉛鉱中に紫色の輝線を示す新元素をみつけ、フランスのラテン名Galliaにちなんで名づけた。ロシアのメンデレーエフが1869年に予言したエカアルミニウムにあたる。分散元素で、天然に単体としては存在せず、希産ではあるが広く分布している。アルミニウム、亜鉛抽出の副産物として得られる。高純度金属は、帯融解法により精製する。軟らかい青みを帯びた白色金属で、融点が低く、液体の温度範囲が広く、固化に際して膨張する。低融点のため塵埃(じんあい)などが付着すると暗色の液体となっていることが多い。アルミニウムに似て金属は酸、水酸化アルカリ水溶液に溶ける。低温では酸化物皮膜により安定であるが、高温では酸素と反応して酸化ガリウム(Ⅲ)を生じる。酸化物は両性で酸化アルミニウムより酸性が強い。酸化数Ⅲの化合物が普通であるが、Ⅰの酸化物、ハロゲン化物も知られる。酸化数Ⅱの化合物と思われるものは、たとえばGaCl2はGaIGaIIICl4のようにⅠとⅢの共存する化合物である。金属間化合物とくにヒ素、アンチモン、リンとの化合物は半導体の性質があり、発光素子、ミリ波、マイクロ波の発振素子などのほか、高温での整流器、トランジスタ、太陽電池などに用いられ、酸化物はカラーテレビの緑色蛍光体として、金属は液体合金の製造に用いられる。[守永健一・中原勝儼]
-
解説
Ga.原子番号31の元素.電子配置1s22s22p63s23p63d104s24p1の周期表13族元素.原子量69.72.2種類の安定同位体と11種類の放射性同位体(69Ga,71Ga)が知られている.1875年P.E. Lecoq de Boisbaudranがせん亜鉛鉱の分光分析で発見し,かれの母国で鉱石の産地フランスの旧名Galliaから元素名をとった.天然には,亜鉛鉱石中やケイ酸塩中に微量含まれている.地殻中の存在度18 ppm.塩化ガリウムの融解電解か,酸化ガリウムを水素で還元して得られる.帯青白色の軟らかい金属.斜方晶系.融点29.78 ℃,沸点2403 ℃.密度5.904 g cm-3(25 ℃),6.1 g cm-3(30 ℃,液体).空気中で安定で,水にも侵されない.酸には水素を発生し,塩をつくって溶ける.常温で塩素,臭素と化合し,熱すればヨウ素,酸素,水素とも結合する.強熱すればアンモニア,硫黄,硫化水素とも反応する.高温では燃焼してGa2O3となる.ほかの金属を侵し合金をつくって溶ける.保存容器として使える材料はタングステン,タンタルのみである.高温用温度計,低融点合金の成分,半導体の製造などに用いられる.67Ga はガリウムシンチレーション法に用いられる.[CAS 7440-55-3][別用語参照]ガリウム化合物
森北出版「化学辞典(第2版)
-
構造
ガリウムは他の金属と異なり、単純な結晶構造のいずれにおいても結晶化しません。常圧状態での安定相としては異なる条件下で形成されるα、β、γ、δ-ガリウムがあり、高圧状態において形成されるものとしては、 Ga-II、Ga-III、Ga-IV が存在します。
1. α-ガリウムの構造
α-ガリウムは、通常の状態下で存在するガリウムの多形で、単位格子に8つの原子が存在する斜方晶系の構造です。最も近い原子同士の距離は244pmで、6つの隣接する原子とはさらに39pm離れています。この不安定な対称性の低い構造が、ガリウムの融点の低さの原因となっていると考えられています。
2. その他の多形
他の結晶形のガリウムは、過冷却状態の液体ガリウムからの結晶化によって得ることができます。−16.3℃以上でガリウム原子がジグザグに並列した構造の単斜晶系β-ガリウムが形成され、−19.4℃以上では12個のガリウム原子が歪んだ形で配列した、α-ホウ素と同様の結晶構造の三方晶系δ-ガリウムが形成されます。−35.6℃では、7個のガリウム原子が環状に配列し、その中央に直鎖型に配列した原子が相互に接続したような構造の斜方晶系γ-ガリウムが形成されます。
-
製法
ガリウムは、他の金属の鉱石の処理中の副産物としてのみ生産され、その主な原料は、アルミニウムの主要な鉱石であるボーキサイトですが、硫化亜鉛鉱石からも抽出できます。バイヤー法ではボーキサイトからアルミナへの処理中に、ガリウムは水酸化ナトリウム液に蓄積するため、イオン交換樹脂の使用後、電気分解によりガリウム金属が得ることができます。半導体用には、ゾーンメルト法や溶融物からの単結晶抽出によりさらに精製され、99.9999%といった非常に高い純度が日常的に達成され、市販されています。
-
説明
Gallium is the 32nd most abundant element and constitutes
0.0005% of the Earth’s crust. It is found most commonly in
association with zinc, germanium, and aluminum and is found
primarily in the mineral germanite. Gallium(III) is the primary
oxidation state for gallium compounds; its chemistry resembles
that of aluminum(III).
-
化学的特性
Gallium is a lustrous, silvery liquid, metal, or gray solid.It is a silvery liquid at 29.75 °C (85.55 °F), which boils at 2204 °C (3999.2°F) and has the largest liquid range of any metal. Despite the liquid state, the vapor pressure of elemental gallium is negligible. Indium is a silvery-white, malleable metal that melts at 156 °C (312.8 °F) and boils at 2072 °C (3761.6°F). Oxides of both elements are amphoteric. Both gallium and indium form arsenides, halides, hydroxides, oxides, and phosphides, some of which may be degraded by acids and fire to produce highly toxic gases such as arsine and stibine. Reaction of indium oxide with water produces an insoluble indium hydroxide [In(OH)3], which limits mobilization in solution. Gallium salt solubility increases with increasing ionic strength.
-
物理的性質
Gallium is soft and bluish off-white when solid and silvery in color as a liquid. It is softenough to cut with knife and has an extremely low melting point. When held in the hand, itwill melt from body heat as it becomes mirror-like in color. It expands when changing backfrom a liquid to a solid. When cold, it becomes hard and brittle. Of all the metals, galliumexhibits the largest range of temperatures from its liquid phase to its solid phase, and, likewater, it expands when it freezes. Its melting point is 29.76°C, its boiling point is 2,204°C,and its density is 5.903 g/cm3,.
-
同位体
There are 33 isotopes of gallium, two of which are stable. They are Ga-69, whichmakes up 60.108% of the element’s presence in the Earth’s crust, and Ga-71, which contributes39.892% of the gallium found in the Earth’s crust. All the other 31 isotopes areradioactive with half-lives ranging from a few nanoseconds to about 15 hours.
-
名前の由来
Latin word Gallia, meaning “Gaul,” an early name for France.
-
天然物の起源
Gallium is the 34th most abundant element, but it is not widely distributed as an elementalmetal. It is usually combined with other elements, particularly zinc, iron, and aluminum ores.It is found in diaspore, sphalerite, germanite, gallite, and bauxite. Although small amounts arerecovered from burning coal used for heating or generation of electricity, it is mostly recoveredas a by-product from the production of ores of other metals. Gallium is about as abundant aslead in the Earth’s crust.
Since 1949, the Aluminum Company of America has extracted gallium metal from aluminumbauxite ore. In the past gallium had few uses. Only recently, with the development ofmicroprocessors, chips, computer, and the like, has gallium found many profitable uses.
-
特性
Gallium is truly an “exotic” element in that it has so many unusual characteristics. It canform monovalent and divalent as well as trivalent compounds. It is considered a “post-transitionalmetal” that is more like aluminum than the other elements in group 13. It has fewsimilar characteristics to the two elements just below it in group 13 (In and Ti).
Gallium reacts strongly with boiling water, is slightly soluble in alkali solutions, acids,and mercury, and is used as an amalgam. It has some semiconductor properties but only if“doped” with elements in group 14, such as As, P, and Sb. It is also used as a “dope” for othersemiconducting elements.
-
使用
Gallium and gallium compounds have numerous uses in
optoelectronics (e.g., LEDs), telecommunication, aerospace,
and many commercial and household items, for example,
alloys, computers, and DVDs. In addition, gallium is used in
special high-temperature thermometers, in place of mercury,
and in arc lamps.
Medically, gallium alloys are used in dental prostheses,
radioactive gallium has been used to locate bone lesions, and
nonradioactive gallium has been used as an antitumor agent.
Gallium has been used experimentally as an adjunct to cisplatinum
cancer chemotherapy. It has also been used to treat
hypercalcemia and inhibit bone resorption. Gallium maltolate
is under development as a treatment for Paget’s disease.
-
調製方法
Most of the world’s gallium is produced in the United States.
The metal is recovered by controlled electrolysis of the concentrated
alkaline liquors that are by-products of the extraction
of aluminum and zinc from their ores. The purification of
bauxite by the Bayer process results in the concentration of
galliumin the alkaline solutions of an aluminum:galliumratio
of 5000:300. Electrolysis using a mercury electrode gives a
further concentration and further electrolysis using a stainless
steel cathode of the resulting sodium gallate affords liquid
galliummetal.Ultrapure (99.9999%)galliumfor semiconductor
electronics is obtainedby repeated fractional crystallization
of themetal.Gallium is relatively expensive because of its low
concentration inmostminerals and because the metalmust be
extremely pure for most applications.
-
定義
gallium: Symbol Ga. A soft silverymetallic element belonging to group13 (formerly IIIB) of the periodictable; a.n. 31; r.a.m. 69.72; r.d. 5.90(20°C); m.p. 29.78°C; b.p. 2403°C. Itoccurs in zinc blende, bauxite, andkaolin, from which it can be extractedby fractional electrolysis. Italso occurs in gallite, CuGaS2, to anextent of 1%; although bauxite onlycontains 0.01% this is the only commercialsource. The two stable isotopesare gallium–69 and gallium–71;there are eight radioactive isotopes,all with short half-lives. The metal has only a few minor uses (e.g. as anactivator in luminous paints), but galliumarsenide is extensively used as asemiconductor in many applications.Gallium corrodes most other metalsbecause it rapidly diffuses into theirlattices. Most gallium(I) and some gallium(II) compounds are unstable. Theelement was first identified by PaulLecoq de Boisbaudran (1838–1912) in1875.
-
一般的な説明
GALLIUM is a silvery-white liquid at room temperature. Ingestion of GALLIUM may be toxic. GALLIUM is corrosive to aluminum. If exposed to high temperatures, GALLIUM may emit toxic fumes which may form a corrosive alkaline solution with water. GALLIUM is soluble in most acids and alkalis. GALLIUM is used as a semiconductor material.
-
空気と水の反応
Stable in dry air, in moist air GALLIUM tarnishes
-
反応プロフィール
Metals, such as GALLIUM METAL, are reducing agents and tend to react with oxidizing agents (i.e. hydrogen peroxide). Their reactivity is strongly influenced by their state of subdivision: in bulk they often resist chemical combination; in powdered form they may react more rapidly. Reacts violently with chlorine and other halogens at ambient temperatures [Bretherick, 5th Ed., 1995].
-
危険性
Most gallium compounds are toxic, particularly the metal gallium arsenide. When forms ofgallium are used in the electronics industry, great care must be taken to protect workers.
-
健康ハザード
Inhalation of vapors or contact with substance will result in contamination and potential harmful effects. Fire will produce irritating, corrosive and/or toxic gases.
-
火災危険
Non-combustible, substance itself does not burn but may react upon heating to produce corrosive and/or toxic fumes. Runoff may pollute waterways.
-
法規情報
ガリウムは、毒物及び劇物取締法、消防法、化学物質排出把握管理促進法 (PRTR法) など、主要な法規制のいずれにも該当していません。
-
取扱いおよび保管上の注意
取扱い及び保管上の注意は、下記の通りです。
- 容器を密栓し、乾燥した冷暗所に保管する。
- 屋外や換気の良い区域のみで使用する。
- 酸、アルカリ、酸化剤、ハロゲンとの混合は避ける。
- 金属、特にアルミニウムを腐食するため、接触の恐れのある部材に使用しない。
- 使用時は保護手袋、保護眼鏡を着用する。
- 取扱い後はよく手を洗浄する。
- 皮膚に付着した場合は、速やかに水で洗い流す。
- 眼に入った場合は、水で数分間注意深く洗う。
参考文献
-
使用用途
ガリウムは、ヒ素との化合物であるガリウムヒ素 (GaAs、通称ガリヒ素) として化合物半導体であるため、として利用されます。ガリウムヒ素は、レーザープリンター、スーパーコンピューター、携帯電話などの電子製品に使われています。
また、ガリウムと窒素の化合物である、 (GaN) は青色発光ダイオード (LED) に使用されています。青色LEDはブルーレイディスク、LED電球、新型の信号機などに使用されております。
-
応用例(製薬)
Gallium has atomic number 31 in the periodic table of elements. It has a silvery-white colour with a melting point of only 29 C, which means that it melts when held in the hand. It has no known physiological role in the human body, but it can interact with cellular processes and proteins that are normally involved in iron metabolism.
It has been shown that gallium ions predominantly accumulate in the bone and therefore would be a good candidate for radiotherapy of bone cancer. Unfortunately, the radioactive isotope 72Ga has only a half-life of around 14h, which is not long enough for effective radiotherapy. Nevertheless, current clinical developments involve the use of radioactive gallium isotopes as tumour imaging reagents, gallium nitrate in metabolic bone disease, hypercalcaemia and as anticancer drug, as well as up-to-date research in the area of chemotherapeutic applications.
-
工業用途
An elementary metal, symbol Ga, gallium is silvery white, resembling mercury in appearance but having chemical properties more nearly like aluminum. Like bismuth, the metal expands on freezing, the expansion amounting to about 3.8%. Pure gallium is resistant to mineral acids, and dissolves with difficulty in caustic alkali. Commercial gallium has a purity of 99.9%. In the molten state it attacks other metals, and small amounts have been used in Sn Pb solders to aid wetting and decrease oxidation, but it is expensive for this purpose.
Gallium alloys readily with most metals at elevated temperatures. It alloys with tin, zinc, cadmium, aluminum, silver, magnesium, copper, and others. Tantalum resists attack up to 450 C, and tungsten to 800 C. Gallium does not attack graphite at any temperature and silica- base refractories are satisfactory up to about 1000 C.
-
職業ばく露
A potential danger to those involved
in preparing such semiconductor compounds as gallium
arsenide. Used in light-emitting diodes, batteries, and
microwave equipment.
-
発がん性
Gallium has not been tested for its ability to adversely affect reproduction. However, some gallium compounds are teratogenics and produce alterations in reproductive capacity.
Carcinogenesis:Gallium has not been tested for its ability to cause cancer in animals. However, gallium is capable of altering several cellular defense mechanisms involved in carcinogenesis. Genetic and Related Cellular Effects Studies. Concentrations of 480 mM cause DNA inhibition in human lymphocytes .
Other: Neurological, Pulmonary, and Skin Sensitization. Repeated exposure may damage the kidneys. Some gallium compounds may affect the nervous system. It is not known if pure gallium can do this. It is not known whether gallium causes lung damage.
-
環境運命予測
Gallium compounds cannot be oxidized, and atmospheric
transformations would not be expected to occur during transport.
Particulate-phase gallium will be removed from the
atmosphere by wet and dry depositions. Gallium compounds
are expected to exist as ions in the environment and therefore
volatilization from water surfaces is not expected to be an
important fate process.
-
輸送方法
UN2803 Gallium, Hazard class: 8; Labels:
8-Corrosive material.
-
純化方法
Dissolve the metal in dilute HCl and extract it with Et2O. Bubbling H2S through the solution removes many metals, and a second extraction with Et2O frees Ga further from metal impurities, except for Mo, Th(III) and Fe which are largely removed by precipitation with NaOH. The solution is then electrolysed in 10% NaOH with a Pt anode and cathode (2-5A at 4-5V) to deposit Ga, In, Zn and Pb, from which Ga was obtained by fractional crystallisation of the melt [Hoffman J Res Nat Bur Stand 13 665 1934]. Ga is also purified by heating to boiling in 0.5-1M HCl, then heating to 40o in water and pouring the molten Ga with water under vacuum through a glass filter (30-50 Y pore size), to remove any unmelted metals or oxide film. The Ga is then fractionally crystallised from the melt under water. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 837 1963.]
-
Structure and conformation
The space lattice of gallium (Ga) belongs to the orthorhombic system D2h18with lattice constants
a=0.45167 nm, b=0.45107 nm and c=0.76448 nm. A unit cell contains 8 atoms. It is
considered to form the molecular lattice of Ga2, which consists of 3 pairs of atoms with a
bond length of 0.271–0.280 nm and a pair with a short bond length of 0.244 nm.
-
不和合性
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, and epoxides such as lyes, halogens,
and alloys of aluminum. Contact with hydrogen chloride/
hydrochloric acid or hydrogen peroxide may result in
explosion. Corrosive on contact with metals. Moisture,
oxygen, and air sensitive.
-
廃棄物の処理
Use a licensed professional
waste disposal service to dispose of this material. Dissolve
or mix the material with a combustible solvent and burn in
a chemical incinerator equipped with an afterburner and
scrubber. All federal, state, and local environmental regula tions must be observed.