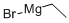Originator
Dolophine ,Lilly,US,1947
Uses
Analgesic (narcotic).
Definition
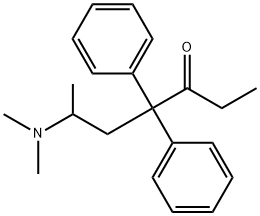
ChEBI: A ketone that is heptan-3-one substituted by a dimethylamino group at position 6 and two phenyl groups at position 4.
Definition
methadone: A synthetic opioid,C
21H
27NO, used medically as an analgesicfor chronic pains and also as asubstitute for heroin in the treatmentof addiction. Methadone is itselfaddictive and considerablequantities of ‘street’ methadone areused in the UK.
Manufacturing Process
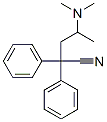
Diphenylacetonitrile is condensed with 2-chloro-1-dimethylaminopropane to give 4-(dimethylamino)-2,2-diphenyl valeronitrile. It is then reacted with ethyl magnesium bromide and then hydrolyzed using HCl to give methadone hydrochloride.
brand name
Dolophine Hydrochloride (Roxane); Dolophine Hydrochloride (Xanodyne);
Methadose (Mallinckrodt); Westadone (Sandoz).
Therapeutic Function
Narcotic analgesic
Biological Functions
Methadone (Dolophine) has an analgesic profile and
potency similar to that of morphine but a longer duration
of action and better oral bioavailability.The kinetic
properties of methadone and its derivative, LAAM,
have been shown to be useful in the treatment of opioid
addiction.
Methadone is a useful analgesic drug for the treatment
of moderate to severe pain. Unlike morphine, it is
generally not used epidurally because of its long duration
of action. It is also rarely or never used in PCA systems
or in pregnant women during labor. The side effects
and signs of overdose following methadone
administration are similar to those observed with morphine.
Overdose is treated with naloxone. Clearance of
methadone is via the urine and bile as the cyclic Ndemethylated
drug. The ability to N-demethylate the
drug decreases in elderly patients, prolonging the action of methadone. In such patients, dosing intervals should
be longer than in younger patients. In addition, the pH
of the urine has a major effect on clearance of the drug.
Alkalinization of the urine or renal insufficiency decreases
excretion of the drug.
Drug interactions and precautions for the use of
methadone are similar to those of morphine. In addition,
rifampicin and hydantoins markedly increase the
metabolism of methadone and can precipitate withdrawal
from methadone. Conversely, the tricyclic antidepressants
and certain benzodiazepines can inhibit
metabolism of methadone, thereby increasing accumulation
of the drug, prolonging its half-life, and intensifying
its side effects. Continuous dosing with methadone
may lead to drug accumulation and to an increased incidence
of side effects; methadone is generally not used
for PCA. In pregnant heroin-addicted women, substitution
of methadone for heroin has been shown to be associated
with fewer low-birth-weight newborns and
fewer learning and cognition problems later in the life
of the child.
General Description
Methadone (Dolophine) is a synthetic opioid approved for analgesic therapy and for the maintenance and treatment of opioid addiction. Methadone is marketed as the racemate, although the opioid activity resides in the R-enantiomer (7–50 times more potent than the S-enantiomer). Methadone may only be dispensed for the treatment of opioid addiction by a program certified by the Federal Substance Abuse and Mental Health Services Administration.
Methadone is a μ-receptor agonist with complex and highly variable pharmacokinetic parameters. The major metabolic pathway of methadone metabolism is via Ndemethylation to an unstable product that spontaneously cyclizes to form the inactive 2-ethylidene-1,5-dimethyl-3,3- diphenylpyrrolidine (EDDP).
Pharmacology
Methadone is a diphenylpropylamine. It has very good oral bioavailability
(~85%) with an oral to parenteral ratio of 1:2. Its plasma half-life can be
highly variable (3–50h, average 24h) but its duration of action is relatively
short. With repeated dosing, problems with accumulation can occur because
of this discrepancy between half-life and analgesic effect. Careful monitoring
is therefore required when converting patients to long-term methadone.
A lso, there is incomplete cross-tolerance with morphine. The racemic
mixture in common use has agonist actions at the MOP receptor (mainly the
laevo isomer) as well as antagonist activity at the NMDA receptor dextro
isomer). Given the importance of this receptor in central sensitisation in a
variety of pain states, there may be cases where methadone offers particular
advantages over and above other opioids (e.g. neuropathic pain).
Plasma concentrations of methadone can be reduced by carbamazepine,
and its metabolism is accelerated by phenytoin.
Safety Profile
Poison by ingestion,
intraperitoneal, intravenous, subcutaneous,
and intraduodenal routes. Human systemic
effects: coma, nausea or vomiting,
respiratory changes, respiratory depression,
somnolence. An experimental teratogen.
Experimental reproductive effects. Caution:
Abuse leads to habituation or addiction.
When heated to decomposition it emits
toxic fumes of NOx. See also
METHADONE HYDROCHLORIDE.