Uses
Used in large amounts in making insulating fi berglass and sodium perborate bleach.
Chemical Properties
Borax is a noncombustible (an inherent
fire retardant), bluish-gray or green, odorless crystalline
powder or granules.
General Description
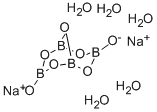
White odorless crystals or free-flowing powder. Density: 1.82 g cm-3. Soluble in water (38 g / L H2O at 20°C). Used in the manufacture of fiberglass insulation and sodium perborate bleach.
Reactivity Profile
Sodium tetraborate pentahydrate is non-combustible. Dissolves somewhat exothermically in water to give basic solutions. Of generally low chemical reactivity. When heated in a closed tube, starts to melt in its own water of crystallization at 128°C and becomes fluid at 140°C. When heated in the open, loses water of crystallization and then melts at 742°C.
Hazard
A severe eye irritant.
Potential Exposure
Borax is used as a soldering flux,
preservative against wood fungus; and as an antiseptic.
Used in ant poisons, for fly control around refuse and
manure piles, as a larvicide. It is used in the manufacture
of enamels and glazes, fiberglass insulation; sodium perborate
bleach; in tanning, cleaning compounds; for fireproofing
fabrics and wood; and in artificial aging of
wood.
Shipping
UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous
hazardous material, Technical Name Required.
Incompatibilities
Dissolves in water forming a basic solution.
Boron dust may form explosive mixture with air.
Contact with strong oxidizers may be violent. Boron is
incompatible with ammonia, bromine tetrafluoride, cesium
carbide, chlorine, fluorine, interhalogens, iodic acid, lead
dioxide, nitric acid, nitric oxide, nitrosyl fluoride, nitrous
oxide, potassium nitrite, rubidium carbide, silver fluoride.
Waste Disposal
Borax, dehydrated: The material
is diluted to the recommended provisional limit
(0.10 mg/L) in water. The pH is adjusted to between 6.5
and 9.1 and then the material can be discharged into sewers
or natural streams.