Synthesis
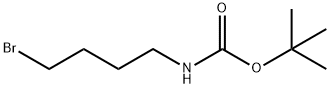
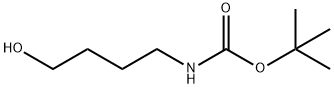
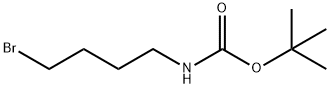
General procedure for the synthesis of tert-butyl N-(4-bromobutyl) carbamate from 4-(N-tert-butoxycarbonylamino)-1-butanol: 4-(N-tert-butoxycarbonylamino)-1-butanol (1.06 g, 5.788 mmol) was dissolved in anhydrous THF (54 mL), followed by the addition of triphenylphosphine (2.86 g, 10.92 mmol, 1.9 equiv) . Carbon tetrabromide (3.62 g, 10.92 mmol, 1.9 eq.) was added slowly with stirring. The reaction mixture was stirred at room temperature for 3 h. After stirring, the reaction mixture was filtered through a diatomaceous earth pad to remove by-products and the filter cake was washed with ether. The filtrate was concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel fast column chromatography (eluent: hexane/ethyl acetate, 3:1) to afford tert-butyl N-(4-bromobutyl)carbamate (1.73 g, quantitative yield) as a white solid.
References
[1] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 2, p. 1151 - 1155
[2] ACS Medicinal Chemistry Letters, 2015, vol. 6, # 11, p. 1156 - 1161
[3] Patent: WO2017/53360, 2017, A1. Location in patent: Paragraph 0154
[4] Patent: WO2017/96045, 2017, A1. Location in patent: Paragraph 00884
[5] Patent: WO2012/9309, 2012, A1. Location in patent: Page/Page column 58