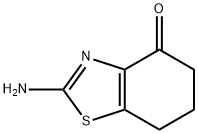
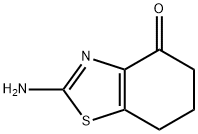
Synthesis
3-Bromocyclohexane-1,2-dione (50 mg, 0.26 mmol) and pyridine-4-thioformamide (24.1 mg, 0.17 mmol) were mixed in ethanol (0.69 mL), heated to reflux and stirred for 15 hours. Upon completion of the reaction, the reaction mixture was diluted with dimethyl sulfoxide (0.5 mL) to ensure that all solids were completely dissolved. Subsequently, the reaction mixture was purified by direct injection into preparative high-performance liquid chromatography (C18 column, water/acetonitrile/ammonium acetate buffer as mobile phase) to afford the target product 2-(pyridin-4-yl)-6,7-dihydrobenzo[d]thiazol-4(5H)-one (30.4 mg, 0.13 mmol, 76% yield) (Table 1, entry 1).
References
[1] Tetrahedron Letters, 2011, vol. 52, # 28, p. 3633 - 3635
[2] Patent: WO2018/81612, 2018, A1. Location in patent: Paragraph 0302
[3] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 19, p. 5879 - 5882