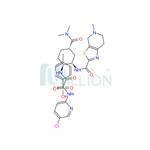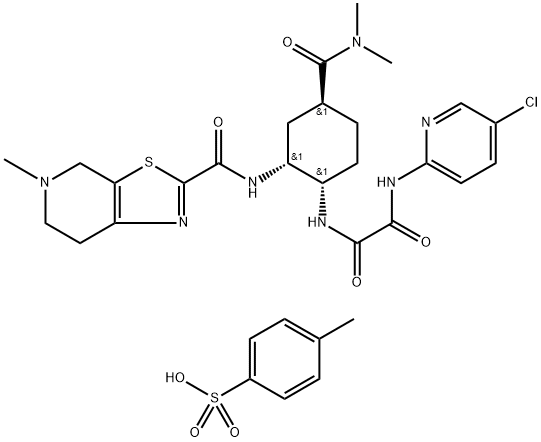
Edoxaban tosylate monohydrate
- Product NameEdoxaban tosylate monohydrate
- CAS1229194-11-9
- CBNumberCB82645582
- MFC31H38ClN7O7S2
- MW720.26
- EINECS1592732-453-0
- MDL NumberMFCD28400751
- MOL File1229194-11-9.mol
- MSDS FileSDS
Chemical Properties
| Melting point | >252°C (dec.) |
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | Chloroform (Very Slightly), DMSO (Slightly), Methanol (Slightly, Sonicated) |
| form | Solid |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | ZLFZITWZOYXXAW-BUZWFPPCNA-N |
| SMILES | S(C1C=CC(C)=CC=1)(O)(=O)=O.N([C@@H]1C[C@@H](C(=O)N(C)C)CC[C@@H]1NC(=O)C(=O)NC1N=CC(Cl)=CC=1)C(C1SC2CN(C)CCC=2N=1)=O |&1:12,14,22,r| |
| FDA UNII | 972203R4EW |
Edoxaban tosylate monohydrate Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| TRC E555525 | 100mg | $195 | EdoxabanTosylateHydrate |
Buy |
| TRC E555525 | 1g | $690 | EdoxabanTosylateHydrate |
Buy |
| Usbiological 448075 | 50mg | $418 | Edoxaban Tosylate Monohydrate |
Buy |
| ApexBio Technology A3383 | 200mg | $680 | Edoxabantosylatemonohydrate |
Buy |
| ChemScene CS-1333 | 50mg | $220 | Edoxabantosylatemonohydrate 99.95% |
Buy |
Edoxaban tosylate monohydrate Chemical Properties,Usage,Production
Synthesis Method
The convergent synthesis of edoxiban tosilate (XI) involves the union of three key structural subunits, diamino cyclohexane intermediate 127, pyridyl amino oxoacetate 128, and thiazole acid 129 (Scheme 1) and the discovery synthesis and related compounds were disclosed in several publications. Several patents on the improved synthesis of the diamino and thiazole intermediates have been disclosed, including an improved synthesis of edoxaban. Because the synthesis described in the latest patents do not involve any chromatographic purification, this is the most likely processscale route, and will be highlighted (Scheme 1–3).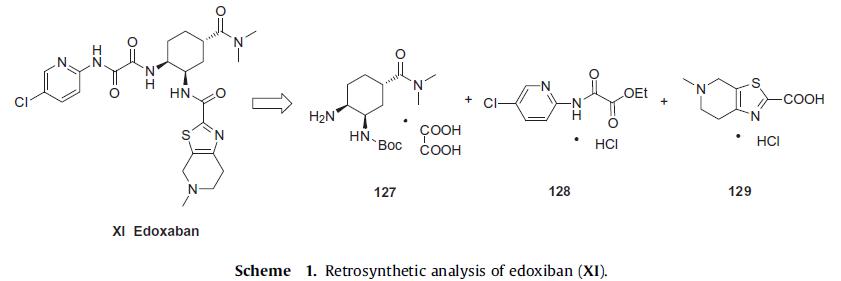
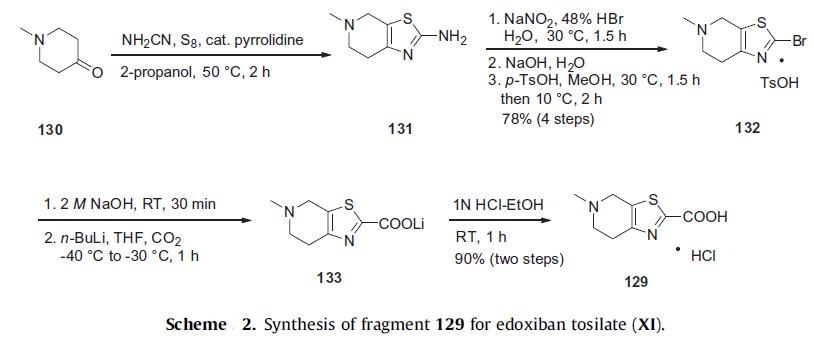
The preparation of the tetrahydropyridyl thiazolo acid 129 is shown in Scheme 2. N-Methyl piperidone (130) is treated with catalytic pyrrolidine, cyanamide, and sulfur in warm isopropanol to yield aminothiazole 131. Diazotization of thiazole amine 131 in the presence of 48% HBr with sodium nitrite at 30°C gave the thiazole bromide, which was directly converted to tosylate salt 132 in 78% yield from piperidone 130. After free-basing the salt with sodium hydroxide, the resulting bromide was treated with n-BuLi followed by bubbling carbon dioxide gas to give lithium carboxylate salt 133. Acidification of this salt in ethanolic HCl gave the desired thiazole acid hydrochloride salt (129) in 90% yield over two steps.
The synthesis of edoxaban tosilate is shown in Scheme 3. Commercially available epoxide ester 134 was reacted with sodium azide to afford the corresponding hydroxyazide intermediate regiospecifically and in quantitative yield. This intermediate was immediately subjected to catalytic hydrogenation in the presence of Boc2O to provide alcohol 135 in 68% yield. Alcohol 135 was converted to the corresponding mesylate, followed by the treatment with sodium azide to give primarily cis-azide 136 in 32% yield over two steps after separation of diastereomers. Reduction of the azide followed by a Cbz protection provided carbamate 137 in 67% yield over two steps. The ester group of 137 was hydrolyzed with lithium hydroxide and reacted with dimethylamine to furnish N,N-dimethylamide 138 in 76% yield over the two step sequence. The Cbz group of 138 was then removed by catalytic hydrogenation and the resulting amine was immediately converted to oxalate salt 127.
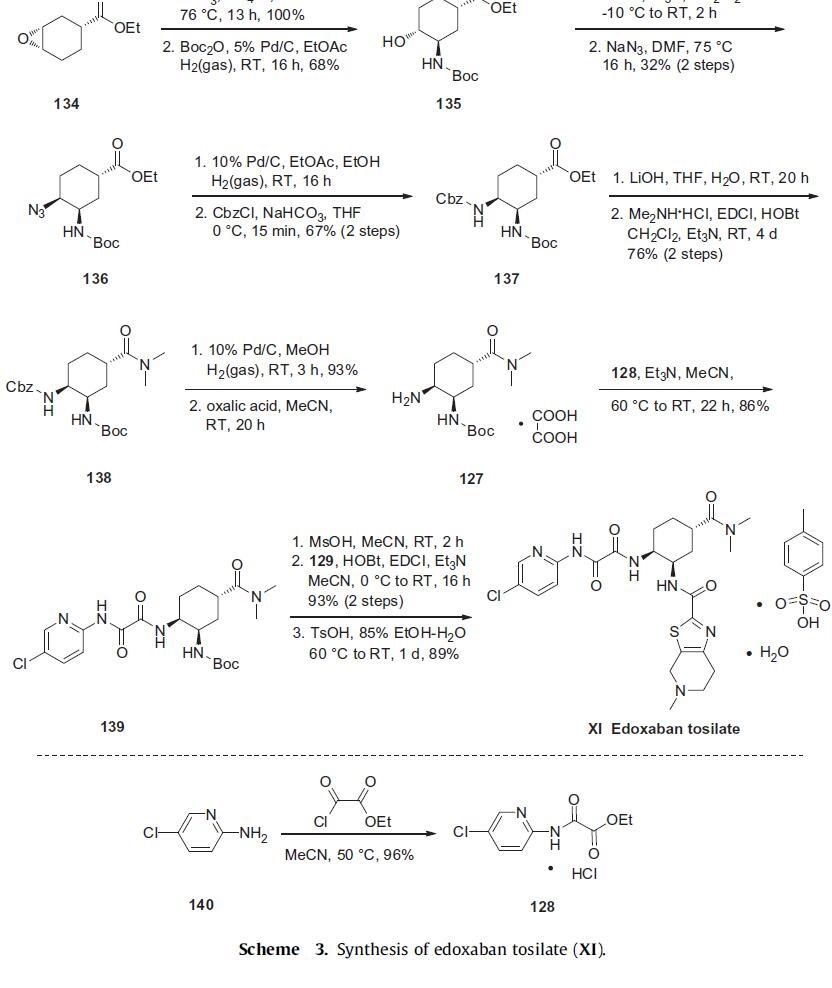
Amine 127 was then condensed with the key pyridylamino oxoacetate 128, prepared in 96% yield by reacting 5-chloropyridin-2-amine (140) with ethyl 2-chloro-2-oxoacetate in warm acetonitrile, to provide amide 139 in 86% yield. Finally, removal of the Boc group of 139 with methanesulfonic acid and EDCI-mediated coupling with tetrahydropyridyl thiazolo acid 129 gave edoxaban in 93% yield. Treatment with TsOH led to isolation of edoxaban tosilate (XI).
Description
Edoxaban tosylate monohydrate is an orally active, highly potent, selective, and direct Factor Xa (FXa) inhibitor with Kis of 0.561 and 2.98 nM for free human FXa and prothrombinase. Edoxaban monohydrate exhibits more than 10,000-fold selectivity over other coagulation proteases. Edoxaban monohydrate can be used for preventing thromboembolic disease research.Uses
Edoxaban is an anticoagulant drug which acts as a direct factor Xa inhibitor.Definition
ChEBI: A hydrate that is the monohydrate of the tosylate salt of edoxaban. Used for the treatment of deep vein thrombosis and pulmonary embolism.Biological Activity
factor xa (fxa), a key serine protease, is a promising target enzyme for the prophylaxis and treatment of thromboembolic diseases. edoxaban tosylate monohydrate is a novel antithrombotic agent that directly inhibits fxa activity.Clinical Use
Daichi Sankyo’s edoxaban tosilate is an orally administered coagulation factor Xa inhibitor that was approved and launched in Japan for the preventive treatment of venous thromboembolic events (VTE) in patients undergoing total knee arthroplasty, total hip arthroplasty, or hip fracture surgery. Edoxaban has been shown to have a rapid onset of anticoagulant effect due to short Tmax (1–2 h) after dosing and sustained for up to 24 h post-dose. Marketed under the brand name Lixiana, it is currently in phase III studies in the US for the prevention of stroke and systemic embolic events in patients with atrial fibrillation (AF) and venous thromboembolism (VTE).in vitro
edoxaban tosylate monohydrate (du-176b) inhibited fxa with ki values of 0.561 nm for free fxa, 2.98 nm for prothrombinase, and exhibited >10 000-fold selectivity for fxa. du-176b doubled prothrombin time and activated partial thromboplastin time in human plasma. du-176b did not impair platelet aggregation by adp, collagen or u46619 [1].in vivo
du-176b dose-dependently inhibited thrombus formation in rat and rabbit thrombosis models, although bleeding time in rats was not significantly prolonged at an antithrombotic dose [1].Drug interactions
Potentially hazardous interactions with other drugsAnalgesics: increased risk of bleeding with NSAIDs and high dose aspirin; increased risk of haemorrhage with IV diclofenac and ketorolac - avoid
Anti-arrhythmics: concentration increased by dronedarone (reduce edoxaban dose)
Antibacterials: concentration increased by erythromycin (reduce edoxaban dose); concentration reduced by rifampicin.
Anticoagulants: increased risk of haemorrhage with other anticoagulants - avoid.
Antidepressants: concentration possibly reduced by St John’s wort.
Antiepileptics: concentration possibly reduced by carbamazepine, fosphenytoin, phenobarbital, phenytoin and primidone.
Antifungals: concentration increased by ketoconazole (reduce edoxaban dose).
Ciclosporin: concentration of edoxaban increased (reduce edoxaban dose).
Metabolism
Unchanged edoxaban is main form in plasma. Edoxaban is metabolised via hydrolysis (mediated by carboxylesterase 1), conjugation or oxidation by CYP3A4/5 (<10%). Edoxaban has 3 active metabolites, the predominant metabolite (M-4), formed by hydrolysis, is active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5%. Edoxaban is a substrate for the efflux transporter P-glycoprotein (P-gp), but not a substrate for uptake transporters such as organic anion transporter polypeptide OATP1B1, organic anion transporters OAT1 or OAT3 or organic cation transporter OCT2. Its active metabolite is a substrate for OATP1B1.Renal clearance accounts for approximately 35% of the administered dose. Metabolism and biliary/intestinal excretion account for the remaining clearance.
References
[1] furugohri t, isobe k, honda y, kamisato-matsumoto c, sugiyama n, nagahara t, morishima y, shibano t. du-176b, a potent and orally active factor xa inhibitor: in vitro and in vivo pharmacological profiles. j thromb haemost. 2008;6(9):1542-9.[2] bathala ms, masumoto h, oguma t, he l, lowrie c, mendell j. pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor xa inhibitor, in humans. drug metab dispos. 2012;40(12):2250-5.
Preparation Products And Raw materials
Edoxaban tosylate monohydrate Supplier
Global(254)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | ||
|---|---|---|---|---|---|---|
| +8618928027945 | sales3@zhhairuide.com | China | 97 | 58 | ||
| 0531-68659554 +8613031714605 |
info@millionpharm.com | China | 294 | 58 | ||
| +86-18600796368 +86-18600796368 |
sales@sjar-tech.com | China | 485 | 58 | ||
| +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 | ||
| +86-0533-2091136 +8613864437655 |
ziboweibinmaoyi@163.com | China | 100 | 58 | ||
| +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 | ||
| +86-371-+86-371-66670886 | info@dakenam.com | China | 19801 | 58 | ||
| 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 | ||
| +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21630 | 55 |
View Lastest Price from Edoxaban tosylate monohydrate manufacturers
Edoxaban tosylate monohydrate Spectrum
1229194-11-9, Edoxaban tosylate monohydrateRelated Search
- (1R,2R,4S)-2-[(tert-butoxycarbonyl)aMino]-4-[(diMethylaMino)carbonyl]cyclohexyl Methanesulfonate
- EthanediaMide, N1-(5-chlo...
- Thiazolo[5,4-c]pyridine, 4,5,6,7-tetrahydro-5-methyl-
- Tert-Butyl(1R,2S,5S)-2-azido-5-[(dimethylamino)carbonyl]cyclohexylcarbamate oxalic acid
- Edoxaban (TsOH salt)
- Edoxaban
- Edoxaban Impurity 12 (1R,2R,4S)
- EthanediaMide, N1-(5-chloro-2-pyridinyl)-N2-[(1S,2R,4R)-4-[(diMethylaMino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-Methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]aMino]cyclohexyl]-
- EthanediaMide, N1-(5-chlo...
- Edoxaban-d6
- Edoxaban Impurity 16
- (1S,3S,4S)-3-AMino-4-hydroxy-cyclohexanecarboxylic acid ethyl ester
- EthanediaMide, N1-(5-chloro-2-pyridinyl)-N2-[(1S,2S,4R)-4-[(diMethylaMino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-Methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]aMino]cyclohexyl]-
- (1S,3R,4S)-4-(2-((5-chloropyridin- 2-yl)amino)-2-oxoacetamido)-3-(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxamido)cyclohexane-1-carboxylic acid
- (1R,3R,4R)-3-AMino-4-hydroxy-cyclohexanecarboxylic acid ethyl ester
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine