Description
Tivozanib (AV-951) is a potent and selective VEGFR inhibitor for VEGFR1/2/3 with IC50 of 30 nM/6.5 nM/15 nM, and also inhibits PDGFR and c-Kit, low activity observed against FGFR-1, Flt3, c-Met, EGFR and IGF-1R. Phase 3.
In vitro
AV-951 is a novel quinoline-urea derivative. AV-951 blocks VEGF-dependent activation of mitogen-activated protein kinases and proliferation of endothelial cells.
In vivo
In vivo studies show that AV-951 also decreases the micro vessel density and suppresses VEGFR2 phosphorylation levels in tumor xenografts, especially at a concentration of 1mg/kg (p.o. administration). AV-951 shows almost complete inhibition of tumor xenografts growth (TGI>85%) in athymic rats. Another study in rat peritoneal disseminated tumor model shows that AV-951 could prolong the survival of the tumor-bearing rats with the MST of 53.5 days. AV-951 displays antitumor activity against many human tumor xenografts including lung, breast, colon, ovarian, pancreas and prostate cancer.
Characteristics
Primary targets: VEGFR/multikinase
Class: receptor tyrosine kinase
Treatment: RCC
Elimination half-life = 111 h
Protein binding = >99%
Uses
Tivozanib is known as an oral VEGF receptor tyrosine kinase inhibitor, exhibiting antitumor effects towards renal cell carcinoma.. Tivozanib suppresses angiogenesis by selectively inhibiting against vascular endothelial growth factor. Potent VEGFR inhibitor.
Uses
Tivozanib also known as AV-951 is an orally bioavailable potent VEGFR-1, 2 and 3, c-Kit and PDGFR inhibitor with IC50 of 0.21, 0.16, 0.24, 1.63 and 1.72 nM, respectively.
Definition
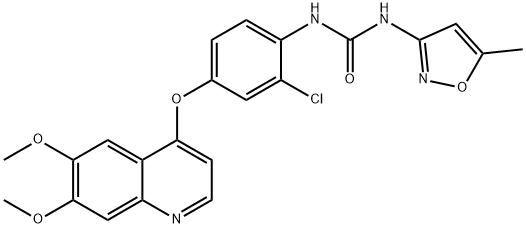
ChEBI: 1-[2-chloro-4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-3-(5-methyl-3-isoxazolyl)urea is an aromatic ether.
Biological Activity
Tivozanib (KRN951) is an oral, next-generation vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI). Tivozanib is a potent, selective inhibitor of VEGFRs 1, 2, and 3 with a long half-life designed to improve efficacy and tolerability.
Mechanism of action
Tivozanib is a tyrosine kinase inhibitor that exerts its actions by inhibiting the phosphorylation of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2 and VEGFR-3 and inhibits other kinases such as c-kit and platelet derived growth factor beta (PDGFR β).
Side effects
- diarrhea
- nausea
- vomiting
- fatigue
- loss of appetite
- weight loss
- voice hoarseness
- back pain
- mouth sores
- cough
- shortness of breath
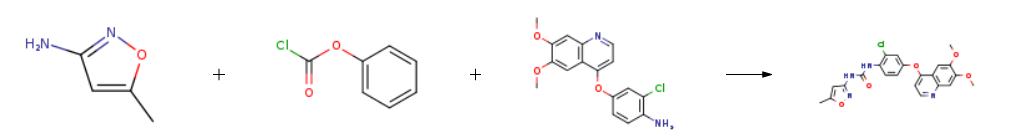
Synthesis
The synthesis of Tivozanib is as follows:
Phenyl chlorocarbonate (601 g) was added dropwise to 3-amino-5-methylisoxazole (377 g), pyridine (1215 g), and N,N-dimethylacetamide (4 L) at 0°C, and the mixture was stirred at 20°C for 2 hr. 4-[(4-Amino-3-chlorophenol)oxy]-6,7-dimethoxyquinoline (847 g) was added to the reaction solution, and the mixture was stirred at 80°C for 5 hr. The reaction solution was cooled to 5°C. Thereafter, methanol (8.5 L) and water (8.5 L) were added thereto, and the mixture was neutralized with an aqueous sodium hydroxide solution. The resultant precipitate was collected by filtration, and the filtered product was slurried in water (8.5 L) for washing. The slurry was filtered, and the filtered product was then dried under the reduced pressure to give Tivozanib (1002 g, yield 86.1%).