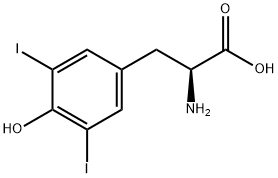
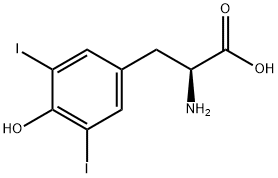
Chemical Properties
DL-3,5-Diiodotyrosine is a lamellar crystal. Decomposed at about 200℃. Solubility in water: 0.149g/L at 0°C, 0.340g/L at 25°C, 0.773g/L at 50°C. Soluble in dilute alkali. Boiled and cooled water and alcohol solution will not produce gel-like precipitate. In contrast, L-3,5-diiodotyrosine crystallizes and dissolves after boiling in dilute alcoholic solution, and a gel-like precipitate is formed by continued boiling.
Uses
3,5-Diiodo-DL-tyrosine is used for diagnosis of hypothyroidism caused by DEHAL1 gene defects.
Synthesis
General procedure for the synthesis of 2-amino-3-(4-hydroxy-3,5-diiodophenyl)propionic acid from D-tyrosine: D-tyrosine (2.00 g, 11.0 mmol) was dissolved in a mixed solution of ice acetic acid (14 mL) and concentrated hydrochloric acid (8 mL). Subsequently, iodine (2.8 g, 11.0 mmol) was added to this solution. A 30% hydrogen peroxide solution was slowly added dropwise to the reaction system at 65 °C. The temperature was maintained and the reaction mixture was stirred continuously for 30 minutes. Upon completion of the reaction, the solution was cooled to 15 °C and diluted with distilled water (100 mL). The pH of the mixture was adjusted to 5 with ammonia to promote precipitation of the target product. The precipitate was collected by filtration and washed with distilled water to give the final brown solid product 2-amino-3-(4-hydroxy-3,5-diiodophenyl)propionic acid in 80% yield. The melting point of the product was >250°C; 1H-NMR (300 MHz, DMSO-D2O) data were as follows: δ 7.46 (s, 2H, Ar-H), 4.10 (t, 1H, -CH-), 2.93 (m, 2H, -CH2-).
References
[1] European Journal of Medicinal Chemistry, 2018, vol. 143, p. 1325 - 1344
[2] Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1907, vol. 51, p. 66
[3] Journal of the American Chemical Society, 1943, vol. 65, p. 1430
[4] Journal of the Chemical Society, 1949, p. Spl. 185, 188
[5] Chemische Berichte, 1935, vol. 68, p. 1108,1115