Antidepressant
Vortioxetine HBr is a new type of diaryl sulfanyl amine antidepressants developed by Japan Takeda and Denmark Lundbeck for the treatment of depression and anxiety. FDA was admitted to be on American market in September 2013 with trade name Brintellix used for the treatment of severe depression in adults and in October of the same year, the admittance application of Vortioxetine to be listed (MAA) received positive comments from European Medicines Agency (EMA) Human Medicines Products Committee (CHMP). CHMP advised to approve of Brintellix being used for the treatment of adult patients with severe depression (MDD). The EMA European Commission granted Vortioxetine the right to be sold throughout the EU in December 2013 with four dose forms,5 mg, 10 mg, 15 mg and 20 mg. Vortioxetine hydrobromide now has applied to be listed in a number of countries except in China.
Vortioxetine HBr is considered to be a new type of multi-model antidepressant. Vitro researches show that it can antagonize 5-HT3,5-HT7and 5-HT1D receptor, activate 5-HT1A receptor, partly activate 5-HT1B receptor and inhibit 5-HT from operation.
Vortioxetine is another kind of antidepressant made by Lundbeck aiming at replace Citalopram which is expired patent drug.
The pharmacological effect, indication, drug interaction, synthetic method and so on are edited by Chemicalbook's Yao Yao.
Vortioxetine
Vortioxetine is an oral immediate release tablet whose main active ingredient is Vortioxetine hydrobromide, which is a kind of antidepressant. Vortioxetine hydrobromide is a slightly yellowish white powder and slightly soluble in water. It is a kind of tablet with different dose forms with each piece containing Vortioxetine hydrobromide 6.335mg,12.71mg,19.065mg or 25.42mg equivalent to 5mg, 10mg, 15mg and 20mg Vortioxetine respectively. The non-active ingredients in Vortioxetine tablet include mnnitol, microcrystalline cellulose, hydroxypropylcellulose, sodium carboxymethyl starch, magnesium stearate and thin film cover made up of hydroxypropylmethylcellulose, titanium dioxide, polyethylene glycol 400, ferric oxide and iron oxide yellow.
Clinical evaluation 1. Vortioxetine has a high affinity for 5-HT. Compared with duloxetine, oxetine, it can act on 5-HT3, 5-HT1A, 5-HT7,5-HT1D and 5-HT1B receptors with high selectivity. When used to regulate emotions , the dose is small, with simple medication once a day improving patient’s compliance. The drug has less drug interactions, high selectivity and less side effects.
Vortioxetine is a new antidepressant and is considered as the most successful drug in the study of single-phase emotional disorder. The results show that Vortioxetine is a more effective antidepressant compared with placebo and can significantly increase 5-HTT possession rate. It is safer with safety similar to venlafaxine and the incidence of adverse reactions is lower than duloxetine. Another clinical study shows that Vortioxetine can effectively reduce the probability of recurrence after treatment.
Animal experiments show that Vortioxetine has a significant effect on behavioral analysis of antidepressant activity. In a number of rat anti-anxiety models, Vortioxetine has higher anti-anxiety activity compared to other known antidepressants and still has effect on the anxiety state on which paroxetine and duloxetine. cannot work.
In summary, compared with other antidepressants, Vortioxetine has high efficacy, less adverse reactions and obvious clinical advantages.
Biological activity
Vortioxetine (Lu AA21004) HBr is a 5-hydroxytryptamine inhibitor that inhibits 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptors and SERT with IC50 being 15nM,33nM, 3.7nM,19nM and1.6nM.
Approval
Vortioxetine hydrobromide is an atypical antidepressant made by Lundbeck and Takeda. It was approved by the United States Federal Drug Agency and the Committee for Medicinal Products for Human Use (CHMP) on September 30, 2013 and December 27, 2013 respectively, for the treatment of major depressive disorder in adults. Vortioxetine has also been investigated as a treatment for generalized anxiety disorder.
In vitro
Lu-AA21004 inhibits recombine human CYP1A2, CYP2C9, CYP2D6 and CYP3A4 with IC50 being 40μM, 39μM, 9.8μM and 10μM respectively. Lu AA21004 is a h5-HT1B receptor agonist with EC50 being 460nM and in the whole cell detection the intrinsic activity is 22%. In vitro full-cell cAMP assay, Lu AA21004 is a functional antagonist against r5-HT7 receptor with Ki being 200nM. Lu AA21004 is a functional antagonist of r5-HT7 receptor with IC50 being 2μM.
In vivo
The clearance on rat liver and oral bioavailability of Lu-AA21004 are 7.1(L/h)/kg and 16% respectively. Lu-AA21004 is injected subcutaneously into the hippocampus of the conscious rats at 2.5mg/kg, 5mg/kg or 10 mg/ kg to increase extracellular 5-HT levels. Lu-AA21004 significantly increases the basal level of 5-HT after being injected into medial prefrontal cortex (mPFC) at the dose of 5mg/kg or 10mg/kg for 3 days.
LuAA21004 affects the Bezold-Jarisch reflection of rats, which is dose-dependent and inhibits transient bradycardia with ED50 being 0.11mg /kg. LuAA21004 is injected subcutaneously into the medial prefrontal cortex and ventral hippocampus of the rats at the dose of 2.5-10.0mg/kg to increase the levels of extracellular 5-HT, DA, and NA. LuAA21004 is injected subcutaneously into the ventral hippocampus of rats at dose of 5mg/kg to increase extracellular 5-HT levels (200%). Lu AA21004 (10mg/kg) significantly reduces pain in rats. Lu AA21004 increases the level of ACh to 224% and 204% at dose of 5 and 10mg/kg after 20min.
Uses
Vortioxetine Hydrobromide is a multimodal serotonergic agent that inhibits 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptor and SERT (1,2,3).
Definition
ChEBI: Vortioxetine hydrobromide is a hydrobromide obtained by combining vortioxetine with one molar equivalent of hydrobromic acid. Used for treatment of major depressive disorder. It has a role as an antidepressant, an anxiolytic drug, a serotonergic antagonist and a serotonergic agonist. It contains a vortioxetine(1+).
Biological Activity
vortioxetine (lu aa21004) hbr (vortioxetine) is an antidepressant, [1] [2] and a serotonin (5-ht) receptor modulator [1] [3] with ki values of 3.7 nm, 19 nm, 200 nm, 33 nm, 15 nm, and 1.6 nm, to human (h) 5-ht3a receptor, h5-ht7 receptor, rat (r) 5-ht7 receptor, h5-ht1b receptor, h5-ht1a receptor and h5-ht transporter (hsert), respectively, with an ic50 value of 2080 nm to r5-ht7 receptor, and with an ec50 value of 460 nm to h5-ht1b receptor [3].5-ht is important in brain, acting as a neurotransmitter in neuromodulation [4].in hela cells expressing h5-ht1b receptors stably, lu aa21004 displayed a partial agonistic response with an ec50 value of 460±110 nm and an ia of 22%. in a polyclonal hek-293 cell line expressing the r5-ht7 receptor, lu aa21004 bound to the r5-ht7 receptor with a ki value of 200±27 nm and was a functional antagonist at the r5-ht7 receptor with an ic50 value of 2080±18 nm [3].in rats, subcutaneous (sc) administration of lu aa21004 resulted in ed50 values of 0.4 mg/kg and 3.2 mg/kg to the rsert and the r5-ht1b receptor, respectively. lu aa21004 acted as an antagonist of 5-ht3 receptor with an ed50 value of 0.11 mg/kg sc [3]. treatment with vortioxetine at doses of 2.5 and 5 mg/kg prior to the acquisition of novel objects made rats show 29 and 33 s, respectively, as average exploration values [5].
Synthesis
As the picture 2 showed, we have developed a new and practical synthetic route to vortioxetine hydrobromide on a hectogram scale. Adopting commercially available materials, through simple and traditional steps, including nucleophilic substitution, catalytic hydrogenation, and cyclization of the piperazine ring, gave the final product in 63.2% yield over three steps and with 99.3% purity (HPLC). Purification methods for the intermediates involved in the route are also given.
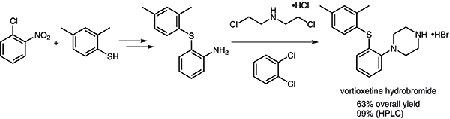
Pic.3 A new synthetic route to vortioxetine hydrobromide
Solubility in water
Vortioxetine hydrobromide is slightly soluble in water; at ambient temperature solubility is equivalent to approximately 1.3 mg base/mL, pH being 5.5 in the saturated solution.
Mode of action
Vortioxetine Hydrobromide is a hydrobromide salt form of vortioxetine, a serotonin (5-HT) modulator and stimulator (SMS), with antidepressant activity. Vortioxetine inhibits the reuptake of serotonin and norepinephrine from the synaptic cleft and acts variably as a serotonin receptor agonist (5-HT1A), partial agonist (5-HT1B) or antagonist (5-HT3, 5-HT1D and 5-HT7). It is not clear how this agent's purported multimodal mechanism of action contributes to its antidepressant effect; however, it is presumed to increase the synaptic availability of serotonin and norepinephrine.
Precautions
Vortioxetine is a serotonergic drug that has a risk of causing serotonin syndrome, especially when combined with other serotonergic drugs such as SSRIs, SNRIs, TCAs, or MAOIs.
Vortioxetine has been known to cause an increase in the risk of bleeding because of interference with serotonin reuptake. The concomitant use of NSAIDs, aspirin, warfarin, and anticoagulants may increase the risk of abnormal bleeding.
Among patients using vortioxetine, reports of symptoms of mania/hypo-mania were less than 0.1%. However, this drug should be used with caution in patients with a personal or family history of bipolar disorder, mania, or hypomania.
Hyponatremia was reported in one patient taking vortioxetine. Hyponatremia is related to the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Elderly patients are at higher risk for hyponatremia, as well as patients taking diuretics. Vortioxetine should be discontinued if signs and symptoms of hyponatremia are present and if there is a decrease in sodium levels[6].
References
[1]. connie sanchez, karen e. asin, francesc artigas, et al. vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. pharmacology & therapeutics, 2015, 145:43-57.
[2]. er-min gu, chengke huang, bingqing liang, et al. an uplc–ms/ms method for the quantitation of vortioxetine in rat plasma: application to a pharmacokinetic study. j. chromatogr. b, 2015, 997:70-74.
[3]. a. mørk, a. pehrson, l.t. brennum, s. møller nielsen, et al. pharmacological effects of lu aa21004: a novel multimodal compound for the treatment of major depressive disorder. j. pharmacol. exp. ther., 2012, 340:666-675.
[4]. david m. lovinger. serotonin’s role in alcohol’s effects on the brain. alcohol health & research world, 1997, 21(2):114-120.
[5]. arne mørk, liliana p. montezinho, silke miller, et al. vortioxetine (lu aa21004), a novel multimodal antidepressant, enhances memory in rats. pharmacology, biochemistry and behavior, 2013, 105:41-50.
[6] Andrew, et al. "Vortioxetine (brintellix): a new serotonergic antidepressant. "P & T : a peer-reviewed journal for formulary management(2015).