Synthesis
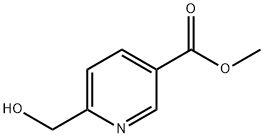
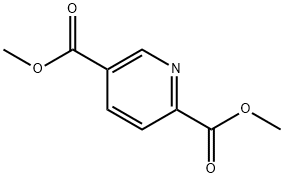
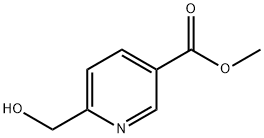
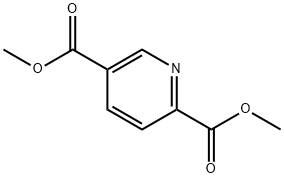
Step 1. Synthesis of methyl 6-(hydroxymethyl)nicotinate (55): a mixture of methyl 2,5-pyridinedicarboxylate (54; 100.0 g, 0.51 mol), CaCl2 (227.4 g, 2.05 mol), and THF (1100 mL) was mixed with EtOH (1200 mL), and stirred for 30 min. NaBH4 (48.6 g, 1.28 mol) was added in batches at 0 °C. The reaction mixture was stirred at room temperature for 18 hours. Subsequently, saturated NH4Cl solution (1.5 L) and water (2.0 L) were added slowly and extracted with dichloromethane (3 x 3.0 L). The organic phases were combined, dried with MgSO4 and concentrated under reduced pressure to give methyl 6-(hydroxymethyl)nicotinate 55 (82.0 g, 96% yield).
References
[1] Patent: WO2010/56549, 2010, A1. Location in patent: Page/Page column 103
[2] Journal of Organic Chemistry, 2010, vol. 75, # 3, p. 650 - 659
[3] Inorganic Chemistry, 2015, vol. 54, # 22, p. 10542 - 10558
[4] Patent: US2010/16373, 2010, A1. Location in patent: Page/Page column 16
[5] Patent: US2016/256578, 2016, A1. Location in patent: Paragraph 0124; 0125