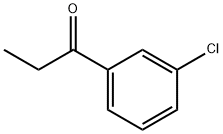
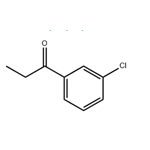
Description
Influence of solvents and temperature on the yield and enantioselectivity of the phenylation of 3′-chloropropiophenone has been investigated.
Chemical Properties
WHITE TO LIGHT YELLOW CRYSTALLINE SOLID
Uses
3'-Chloropropiophenone is a reagent used in the vinylation, alkylation and dienylation of ketones. It is also used in the preparation of thizaine derivatives that show antibacterial activity.
Uses
3'-Chloropropiophenone has been used in the preparation of 1-(3-chlorophenyl)-1-phenyl-1-propanol.
Application
3′-Chloropropiophenone can be used as a reactant to synthesize:
(S)-3-chloro-1-phenylpropanol via bio-catalyzed asymmetric reduction method.
1-(3-Chlorophenyl)-1-phenyl-1-propanol by phenylation with diphenylzinc in the presence of dihydroxy bis(sulfonamide) ligand.
(S)-Dapoxetine, a selective serotonin reuptake inhibitor.
Preparation
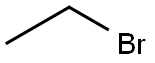
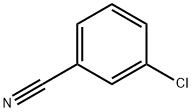
4.86 g (0.2 mol) of magnesium turnings, 30 ml of dry diethyl ether and a grain of iodine are placed in a one liter three-necked round-bottomed flask equipped with a condenser, a calcium chloride drying tube, a pressure equalizing funnel and a magnetic stirrer; the flask is purged with nitrogen, and 21.8 g (0.2 mol) of ethyl bromide in 30 ml of dry diethyl ether are then added. The mixture is then heated under reflux for one hour and left to cool. At ambient temperature, 16.51 g (0.12 mol) of 3-chlorobenzonitrile in 70 ml of dry diethyl ether are then added. A copious precipitate forms. The mixture is stirred overnight at ambient temperture and then cooled in an ice bath and hydrolysed by slowly adding 50 ml of water and then about 100 ml of 6N hydrochloric acid until the pH is acid. The mixture is stirred for one and a half hours and then extracted with ethyl acetate. The organic extract is then washed twice with water, dried and concentrated on a rotary evaporator. This gives 26 g of an orange oil, which is concentrated in vacuo to give about 18.2 g of ochre crystals of 3'-chloropropiophenone melting at about 40° C.
Literature source US04690931