Chemical Properties
Off-White to Pale Yellow Solid
Uses
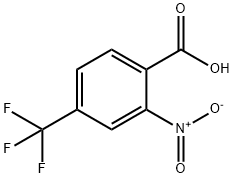
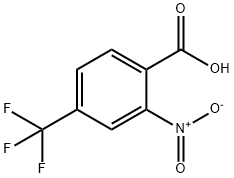
2-Nitro-4-(trifluoromethyl)benzoic Acid (cas# 320-94-5) is a compound useful in organic synthesis.
Synthesis
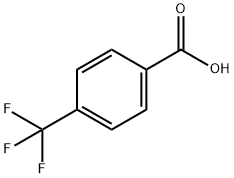
Example 2 a) Synthesis of 2-nitro-4-trifluoromethylbenzoic acid: 11.97 g (63 mmol) of 4-trifluoromethylbenzoic acid was slowly added in batches to 48 mL of concentrated nitric acid (100%) at room temperature. Subsequently, the reaction mixture was heated to reflux for 1 h. After completion of the reaction, it was cooled to room temperature and slowly poured into about 600 g of ice. After stirring the mixture for 1 hour, the precipitate was collected by filtration and washed with 1 L of water. The filtrate was extracted with 300 mL of dichloromethane (CH2Cl2), the organic phase was combined with the precipitate and dried over anhydrous sodium sulfate (Na2SO4). The solvent was removed by distillation under reduced pressure and the residue was recrystallized by dissolving in 1 L of diisopropyl ether (DIP) and adding 2 L of heptane (HEP) at 68 °C, followed by slow cooling of the solution to room temperature. The crystalline product was washed with 1 L of heptane and dried under reduced pressure to give 7.1 g (48% yield) of 2-nitro-4-trifluoromethylbenzoic acid with a melting point of 136 °C - 138 °C.
References
[1] Patent: US2005/124681, 2005, A1. Location in patent: Page/Page column 16