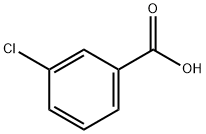
Chemical Properties
white to light yellow crystal powder
Uses
A Pseudomonas species which utilized 3-chlorobenzoic acid as a carbon source converted this compound to 3-hydroxybenzoic and 2, 5-dihydroxybenzoic acids. A methanogenic consortium able to use 3-chlorobenzoic acid as its sole energy and carbon source was enriched from anaerobic sewage sludge. A Pseudomonas putida strain 87 capable of assimilating 3-chlorobenzoic acid as a sole source of carbon and energy (3Cba+) was isolated. coupling of 3-chlorobenzoic acid with phenylboronic acid proceeds at room temperature using 0.5 % Pd and at 100°C using 0.1 % Pd to provide the coupled product in 97 % yeild.
Uses
3-Chlorobenzoic acid is a fundamental chemical building block commonly used in organic synthesis of more complex structures. A benzoic acid analogue that showed antifungal activity against strains of Aspergillus flavus, Aspergillus fumigatus and Aspergillus terreus, causative agents of human aspergillosis, in in vitro bioassays.
Definition
ChEBI: 3-chlorobenzoic acid is a monochlorobenzoic acid carrying a chloro substituent at position 3. It has a role as a drug metabolite. It is functionally related to a benzoic acid. It is a conjugate acid of a 3-chlorobenzoate.
Synthesis Reference(s)
Journal of the American Chemical Society, 76, p. 5755, 1954
DOI: 10.1021/ja01651a039Tetrahedron Letters, 25, p. 783, 1984
General Description
Crystals or fluffy white powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
3-Chlorobenzoic acid is incompatible with strong oxidizing agents. The reactivity of the chlorine atom of 3- chlorobenzoic acid is sluggish compared with the other isomers. Decarboxylation at higher temperatures is insignificant, and it resists oxidation. 3-Chlorobenzoic acid is made by the oxidation of 3-chlorotoluene or by the hydrolysis of 3-chlorobenzoyl chloride, which is derived from benzoyl chloride. 3-Chlorobenzoic acid is used for making diphenyl ether type herbicides.
Fire Hazard
Flash point data for 3-Chlorobenzoic acid are not available; however, 3-Chlorobenzoic acid is probably combustible.
Purification Methods
Crystallise the acid successively from glacial acetic acid, aqueous EtOH and pet ether (b 60-80o). It also recrystallises from *C6H6 or Et2O/hexane, and sublimes at 55o in a vacuum. [Anal Chem 26 726 1954] The methyl ester has m 21o, b 231o/760mm. The S-benzylisothiouronium salt has m 164-165o (from EtOH) [Friediger & Pedersen Acta Chem Scand 9 1425 1955, Samuel J Chem Soc 1318 1960]. [Beilstein 9 IV 969.]