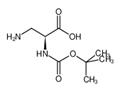
Synthesis
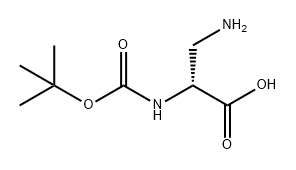
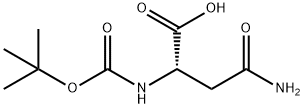
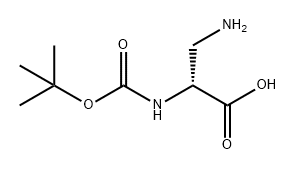
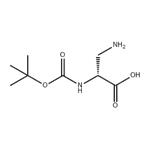
N-Boc-L-asparagine (13; 3.48 g, 15 mmol) was used as starting material, which was co-dissolved with PIDA (5.81 g, 18 mmol) in a solvent mixture of EtOAc, MeCN, and H2O (2:2:1, v/v, total volume 62.5 mL). The reaction was carried out at 16°C for 30 min. Subsequently, the reaction system was slowly warmed to room temperature and stirring was continued for 4 hours. A precipitate was generated during the reaction, which was collected by filtration and dried to afford the target product (R)-3-amino-2-((tert-butoxycarbonyl)amino)propionic acid in a yield of 2.02 g (66% yield, 9.89 mmol) as a colorless solid with a melting point of 211-213 °C (literature value 216 °C). The product was confirmed by 1H NMR (500 MHz, DMSO-d6): δ= 1.40 [s, 9H, C(CH3)3], 2.76 (dd, 3J = 9.2 Hz, 2J = 11.8 Hz, 1H, CH2), 3.06 (dd, 3J = 5.1 Hz, 2J = 11.8 Hz, 1H, CH2), 3.65-3.69 (m 1H, COCH), 6.08 (s, 1H, OCONH). No proton signals were observed for the COOH and NH2 groups.13C NMR (125 MHz, DMSO-d6) data: δ = 28.3, 40.8, 51.1, 78.2, 155.2, 171.2. LC-MS (ESI) analysis showed 100% purity of the product; m/z = 205.19 ([M + H]+), 203.09 ([ M - H]-). HRMS (ESI+) analysis results: calculated value C8H16N2O4 ([M + H]+) m/z = 205.1183; measured value: 205.1177.
References
[1] Organic and Biomolecular Chemistry, 2015, vol. 13, # 6, p. 1629 - 1633
[2] Chemical Communications, 2011, vol. 47, # 4, p. 1198 - 1200
[3] Journal of Organic Chemistry, 2012, vol. 77, # 13, p. 5696 - 5704
[4] Synlett, 2008, # 4, p. 513 - 516
[5] Journal of Peptide Science, 2017, vol. 23, # 3, p. 202 - 214