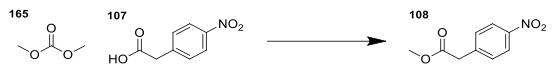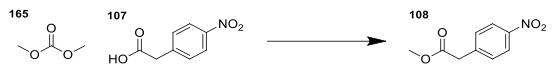Synthesis
4-nitrophenylacetic acid(2.00 g, 11 mmol) was added to a clean
carousel tube. Dimethyl carbonate (3 mL, 3.21 g, 35.6 mmol) was
added, and the reaction was heated at reflux overnight. The brown
solution was allowed to cool, and poured in to aqueous sodium
bicarbonate (10%, 10 mL) while stirring. The resulting mixture was
extracted with ethyl acetate (3 x 10 mL). The combined organic layers
were washed with water and brine (10 mL each), and dried over anhydrous
magnesium sulphate. After filtration and evaporation of the solvents,
proton NMR analysis showed the product had formed, and none of the
precursor acid remained. No further purification was performed. This
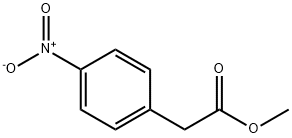
gave methyl p-nitrophenylacetate as a pale orange oil (1.929 g, 9.89
mmol, 90%)): 1H NMR (400 MHz, CDCl 3 ) |? 8.20 (d, J = 8.6 Hz, 2H), 7.46 (d,
J = 8.6 Hz, 2H, 3.74 (s, 2H), 3.73 (s, 3H).