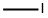Synthesis
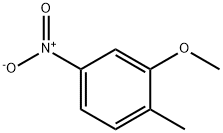
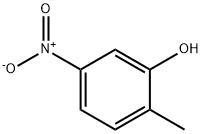
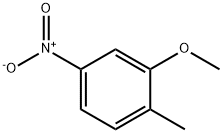
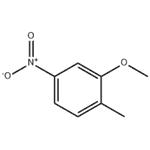
GENERAL METHOD: Under argon protection, 2-methyl-5-nitrophenol (613 mg, 4.0 mmol) was dissolved in anhydrous DMF (7 mL) and potassium carbonate (608 mg, 4.4 mmol) and iodomethane (12.0 mmol) were added sequentially. The reaction mixture was heated with stirring at 40 °C for 2-3 hours. After completion of the reaction, the mixture was poured into water and extracted with ethyl acetate (EtOAc). The organic layers were combined, washed sequentially with water and saturated brine and dried over anhydrous magnesium sulfate (MgSO4). The solvent was removed by concentration under reduced pressure and the resulting residue was purified by silica gel column chromatography to afford the target product 2-methyl-5-nitroanisole.
References
[1] European Journal of Organic Chemistry, 2009, # 27, p. 4614 - 4621
[2] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 10, p. 3156 - 3172
[3] Chemical and Pharmaceutical Bulletin, 2014, vol. 62, # 10, p. 979 - 988
[4] Patent: US2004/214798, 2004, A1. Location in patent: Page 18