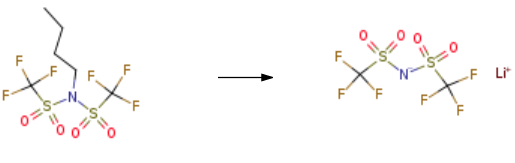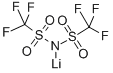
Lithium bis(trifluoromethanesulphonyl)imide
- Product NameLithium bis(trifluoromethanesulphonyl)imide
- CAS90076-65-6
- CBNumberCB6311504
- MFC2HF6NO4S2.Li
- MW288.1
- EINECS415-300-0
- MDL NumberMFCD00210017
- MOL File90076-65-6.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 234-238 °C(lit.) |
| Density | 1,334 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| Flash point | >100°C (>212°F) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 10 mg/mL, clear, colorless |
| form | Hygroscopic Powder |
| Specific Gravity | 1.334 |
| color | White |
| Water Solubility | Soluble in water. |
| Sensitive | Moisture Sensitive |
| BRN | 6625414 |
| InChI | 1S/C2F6NO4S2.Li/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q-1;+1 |
| InChIKey | QSZMZKBZAYQGRS-UHFFFAOYSA-N |
| SMILES | [Li]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F |
| LogP | -1.46 |
| CAS DataBase Reference | 90076-65-6(CAS DataBase Reference) |
| FDA UNII | 9Y730392A5 |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311-H314-H373-H412 | |||||||||
| Precautionary statements | P260-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T,C | |||||||||
| Risk Statements | 24/25-34-52/53-48/22 | |||||||||
| Safety Statements | 22-26-36/37/39-45-61 | |||||||||
| RIDADR | UN 2923 8/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| Hazard Note | Harmful/Corrosive/Moisture Sensitive | |||||||||
| TSCA | TSCA listed | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29309090 | |||||||||
| Storage Class | 6.1B - Non-combustible acute toxic Cat. 1 and 2 very toxic hazardous materials | |||||||||
| Hazard Classifications | Acute Tox. 3 Dermal Acute Tox. 3 Oral Aquatic Chronic 3 Eye Dam. 1 Skin Corr. 1B STOT RE 2 Oral | |||||||||
| NFPA 704: |
|