Synthesis
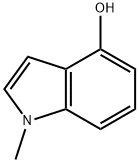
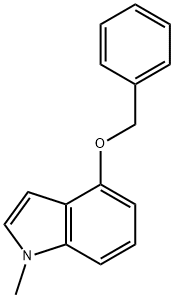
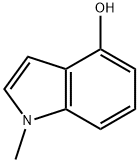
b) Synthesis of 4-hydroxy-1-methylindole: In a hydrogenation unit, 4-(benzyloxy)-1-methyl-1H-indole (0.250 g, 1.12 mmol) was dissolved in methanol (2.25 mL) to form a brown solution. To this solution was added 5% Pd/C catalyst (0.119 g, 1.12 mmol, pre-dried in an oven). The mixture was connected to a hydrogenation unit and after three degassing operations, it was charged with hydrogen. After the last degassing, the reaction was shaken at 40 psi hydrogen pressure for 16 hours. Upon completion of the reaction, the reaction mixture was diluted with methanol (15 mL), filtered through a layer of diatomaceous earth (2.5 inches at a flow rate of 2 L/h) and washed with warm methanol (75 mL). The organic filtrate was concentrated to give 0.160 g (97% yield) of a black oily product. The product was characterized by 1H NMR (CDCl3): δ 7.07 (t, J = 8.24 Hz, 1H), 6.92 (m, J = 8.24 Hz, 2H), 6.52 (m, J = 2.47 Hz, 2H), 4.60 (br s, 1H), 3.75 (s, 3H).
References
[1] Patent: US2006/104998, 2006, A1. Location in patent: Page/Page column 20
[2] Journal of Medicinal Chemistry, 2008, vol. 51, # 3, p. 417 - 423
[3] Patent: CN108358760, 2018, A. Location in patent: Paragraph 0046
[4] Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 17, p. 4090 - 4094
[5] Patent: CN103254191, 2016, B. Location in patent: Paragraph 0134; 0135