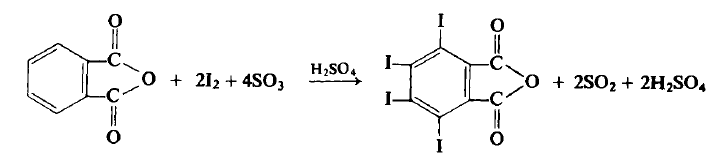
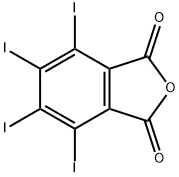
Preparation
CAUTION: This reaction should be carried out in a well-ventilated hood. <br/>To a flask equipped with a mechanical stirrer and an air condenser topped with a tube leading to a gas trap is added 74.0 gm (0.5 mole) of phthalic anhydride, 162 gm (0.638 mole) of iodine, and 300 ml of 60% fuming sulfuric acid (1.84 moles). The flask is gently heated to 45-50°C, at which point the reaction commences. If the reaction becomes too vigorous it may be necessary to use a ice bath to lower the temperature to 40-50°C. The reaction mixture is eventually (4 hr) heated up to 65°C until all visible reaction has ceased. The reaction mixture is cooled to 10-20°C and an additional 81.0 gm (0.318 mole) of iodine is added and the reaction again slowly heated up to 65°C (1?hr) and again when the reaction ceases it is cooled. Another 27.0 gm (0.107 mole) of iodine is added and the reaction is again heated up to 65°C (1 hr). The flask is heated with an oil bath to a bath temperature of 175-180°C, at which point the sulfur trioxide and iodine fumes evolve. After about 2 hr, when the gaseous evolution ceases, the flask is cooled to about 60°C and the mxiture then poured into a beaker of water. The contents are allowed to stand overnight at room temperature, filtered, washed with two 50-ml portions of cone, sulfuric acid and then with three 100 ml portions of water. The light yellow crystalline product is put into a beaker containing 1 liter of water and 10 gm of sodium bisulfite in order to remove the last traces of free iodine. The aqueous solution is decanted, the product washed five times with ? liter of water, washed twice with 100 ml of acetone and then dried at 60°C to afford 260-268 gm (80-82%), m.p. 327-328°C.