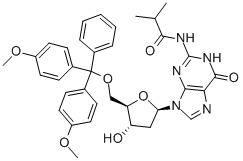
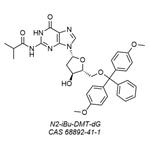
Synthesis
2N-Isobutyryl deoxyguanosine (B5-I) was dried by co-evaporation with dry pyridine three times. To a stirred suspension of dry (B5-I) (14.15 mmol) in pyridine (100 mL), a solution of dimetoxytrityl chloride (14.8 mmol) in pyridine (30 mL) was added dropwise over 60 min. The reaction mixture was left for 4 h at room temperature, cooled to 0 C. by immersion in an ice water bath, quenched with 5% NaHCO3 (100 mL), and extracted with ethyl acetate (3×100 mL). The organic fractions were pooled, dried over magnesium sulfate, and concentrated in a vacuum, and the residue was co-evaporated with toluene. The gum oil residue was dissolved in a minimum amount of methylenechloride and added dropwise to a mixture of ethyl and petroleum ether (2000 mL 75/25) with stirring. After 20 min, pure 5'-O-Dimethoxytrityl-N-isobutyryl-deoxyguanosine precipitated from the solution and was filtered off and dried. Yield: 75%