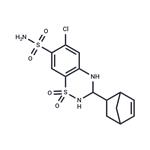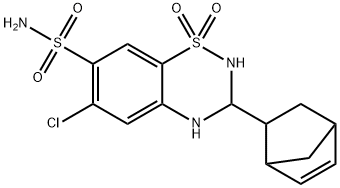
CYCLOTHIAZIDE
- Product NameCYCLOTHIAZIDE
- CAS2259-96-3
- CBNumberCB6123851
- MFC14H16ClN3O4S2
- MW389.88
- EINECS218-859-7
- MDL NumberMFCD00210192
- MOL File2259-96-3.mol
Chemical Properties
| Melting point | 234° |
| Density | 1.3781 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 35 mg/ml) or in Ethanol (up to 9 mg/ml) |
| pka | pKa 9.1(30% EtOH) (Uncertain) |
| form | White to off-white solid. |
| color | Off-white |
| λmax | 273nm(lit.) |
| Merck | 14,2754 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| FDA UNII | P71U09G5BW |
| ATC code | C03AA09 |
| EPA Substance Registry System | 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3-bicyclo[2.2.1]hept-5-en-2-yl-6-chloro-3,4-dihydro-, 1,1-dioxide (2259-96-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H335-H319-H315 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DK9610000 | |||||||||
| HS Code | 2935904000 | |||||||||
| Hazardous Substances Data | 2259-96-3(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral in rat: > 5gm/kg | |||||||||
| NFPA 704: |
|