Synthesis
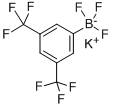
A solution of 3,5-bis(triflfluoromethyl)phenylboronic acid (10 g, 38.8 mmol) in methanol (15 mL) was added to an aqueous solution of KHF2 (4.5 M, 26 mL, 117 mmol) at room temperature. A heavy precipitate was deposited. The resulting suspension was stirred 148 MODERN ORGANIC SYNTHESIS IN THE LABORATORYfor 1 h at room temperature, and the precipitated product was collected and washed with methanol. Recrystallization from a minimum amount of acetone produced 11 g (89%) of potassium 3,5-bis(triflfluoromethyl)phenyltriflfluoroborate. Reference: Molander, G. A.; Biolatto, B. J. Org. Chem. 2003, 68, 4302−4314.
Uses
Thos chemical has been used for inserting a 3,5-bis(trifluoromethyl)phenyl group into the position of a halogen or triflate group of various arenes and various heterocycles in Pd-catalyzed Suzuli-Miyaura cross-coupling reactions.