Description
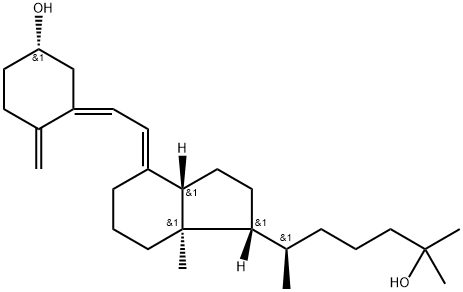
Calcifediol (synonym 25-Hydroxyvitamin D3) is a vitamin D analog and the major metabolite of vitamin D3 (Cholecalciferol) in the liver. Its main function is the regulation of calcium and phosphorus, which is necessary for strong bones. It is used to treat patients with serious kidney diseases to decrease the parathyroid hormone (PTH) level. It is used to treat rickets and osteomalacia, both in azotemic and non-azotemic patient. Calcifediol is sold under RAYALDEE® to treat secondary hyperparathyroidism associated with vitamin D insufficiency in stage 3-4 chronic kidney disease.
Therapeutic drug for bone disease
The natural source of Vitamin D in the body depends on ultraviolet rays of sunlight, making the 7-dehydrocholesterol convert into vitamin D3, at first under the action of vitamin D3 hydroxylase in liver vitamin D3 converts into the Calcifediol, vitamin D3 mainly is present in the form of the Calcifediol in the blood, in the kidney the Calcifediol is further converted to 1,25-dihydroxyvitamin D3 (calcitriol for short) and 24,25-dihydroxyvitamin D3, activity of calcitriol is 2-5 times of the Calcifediol, the two drugs can promote bone re-absorption of calcium and intestinal absorption of Ca2 +. In addition to regulating the body's calcium and phosphorus metabolism, this product can promote the intestinal absorption of calcium and phosphorus, promote calcium and phosphorus deposits in the bone tissue, promote bone formation.
Calcifediol itself has a certain activity, can be used for the treatment of metabolic bone diseases, such as osteoporosis, rickets, osteomalacia, but also for hemodialysis-induced hypocalcemia. Patients with chronic renal failure combined with metabolic bone diseases, engaged during dialysis, can be applied. It can increase the levels of serum calcium, can reduce levels of serum alkaline phosphatase and parathyroid hormone, improve radiological and histological sign of osteitis (with or without osteomalacia). Bone resorption, hyperparathyroidism and osteodystrophy signs of mineralization defects all can be reduced, as for osteomalacia patients and children who are unable to obtain sufficient bone development because of renal osteodystrophy or parathyroid dysfunction, may be more favorable than calcitriol. Such cases can be treated with Calcifediol and calcitriol. But as for osteomalacia accompanied by gastrointestinal diseases, Calcifediol is preferable. If because of liver disease to cause generated obstacles of 25-OHD in vivo, secondary osteomalacia, can also try to use this product. But osteomalacia caused by aluminum poisoning, treatment of vitamin D preparations often is ineffective. Chelator deferoxamine (Desferal) clear aluminum of blood dialysis, can be effective.
Osteomalacia and rickets (in children causing deformities severe osteomalacia) is characterized by obstacle of bone mineralization and non-calcified bone-like tissue or cartilage accumulation. Bone changes of Vitamin D-related bone softening, accompanied with normal or reduced levels of serum calcium and phosphorus, increased serum alkaline phosphatase, and PTH is secondary increased.
Osteoporosis is characterized by reduced bone tissue thereby increasing the likelihood of fractures. Pathological mechanism may be an increased rate of bone resorption, exceeding bone formation; or decreased bone formation, and bone resorption is also still normal or reduced. Bone is progressive missing, can compress and collapse the spine, causing pain, body shorter and kyphosis. Patients are also prone to the outer periphery fracture.
The above information is edited by the chemicalbook of Liu Yujie.
Side effects and precautions
Like all the vitamin D metabolites, excessive Calcifediol can also cause increased serum calcium, in urine can increase high. Therefore, in the dose adjustment period, serum calcium should be monitored at least once a week, if increased serum calcium was found, it should be discontinued.
Pregnant women use this product, there is no adequately controlled studies, but in experimental animals, teratogenic effects have been found.
The US Food and Drug Administration (FDA) classify the product into pregnancy category C drugs.
People who use digitalis, should be used with caution vitamin D, because increased calcium may induce arrhythmia.
Other side effects and precautions, see "Vitamin D2" (Vita-min D2) that is ergocalciferol.
Action of Vitamin D2 is intense, may cause damage. Increased serum calcium caused by excess, can cause gastrointestinal and central nervous system lesions, soft tissue calcification. Kidney complications can be very serious and even cause death. Such as increased serum calcium persists, after discontinuation, kidney damage likely will continue for a long time. An individual who the amount is not too large, but also because of increased sensitivity to occur adverse reactions.
Calcifediol is rapidly absorbed, at 4 to 8 hours up to serum peak concentration. Transport shall bind with protein, half-life is about 16 days.
References
- https://pubchem.ncbi.nlm.nih.gov
- https://de.wikipedia.org/wiki/Calcidiol
- https://www.webmd.com
- https://www.drugbank.ca
Chemical Properties
White Solid
Originator
Dedrogyl,Roussel,France,1976
Uses
A metabolite of Vitamin D. The principal circulating form of vitamin D3, formed in the liver by hydroxylation at C-25. Calcium regulator.
Uses
This product may be used as an analytical standard.
Uses
25-Hydroxyvitamin D
3-23,24,25,26,27-
13C
5?can be used as a stable isotope internal standard for data interpretation in specific biological samples by mass spectrometry.
Definition
ChEBI: A hydroxycalciol that is calciol in which the hydrogen at position 25 has been replaced by a hydroxy group. A prehormone resulting from the oxidation of calciol in the liver, it is further hydroxylated in the kidney to give calcitriol, the active form of v
tamin D3.
Manufacturing Process
A solution of 125 mg of cholesta-5,7-diene-3β,25-diol in 125 ml of benzene
and 10 ml of absolute ethanol is placed in a photo reactor equipped with a
quartz lamp well cooled with water and a nitrogen inlet. The reaction mixture
is cooled to about 16°C, and purged with N2. A Hanovia 8A36, 100-watt lamp,
centered in the lampwell 2.5 cm from the internal surface of the reaction
mixture, is turned on for 15 minutes, including the 5-6 minutes required for
the lamp to reach full brilliance. The lamp is a typical actinic energy source
suitable for the irradiation step in the known synthesis of Vitamin D, and can
be replaced by any such available lamp. The specific lamp used is a 100-watt
high-pressure quartz mercury-vapor lamp, producing approximately 11.5
watts total radiated energy distributed over the range of 220-1400 nm. A fast
stream of water is necessary to keep the outlet water temperature below
20°C. The reaction mixture is concentrated to dryness in a rotary evaporator
below room temperature. The semisolid residue is triturated with 5 ml of 35%
ethyl acetate-65% Skellysolve B hexanes mixture and filtered and another 5
ml of the same solvent is used for wash. The solid contains unreacted starting
material and the liquor contains the product. The liquor is poured onto a 40 g
column containing TLC grade Florisil, 150-200 mesh packed wet with 35%
ethyl acetate-Skellysolve B hexanes, and the products are eluted with the
same solvent mixture collecting 10 ml fractions. The fractions containing the
product, located by spotting on a TLC plate, are combined and evaporated to
dryness below room temperature to give an oily residue. A few drops of
absolute ether are added and removed under vacuum to give 25-
hydroxyprecholecalciferol as a fluffy foam; yield 60 mg.
A solution of about 300 mg of 25-hydroxyprecholecalciferol prepared as
described above in 5 ml of chloroform is heated for 3.5 hours at 70°-75°C
under N2 in a sealed flask. The solvent is evaporated and the residue is
chromatographed through a 60 g column containing TLC grade Florisil, 150-
200 mesh packed wet with 35% ethyl acetate in Skellysolve B hexanes. The
column is eluted with the same solvent mixture, collecting 10 ml fractions.
The fractions which crystallize on trituration with aqueous methanol are
combined and recrystallized twice from aqueous methanol to give 25-
hydroxycholecalciferol hydrate; yield 120 mg, MP 81°-83°C (sinters 75°C).
A solution of 20 mg of 25-hydroxycholecalciferol hydrate, prepared as
described above, in 20 ml of methylene chloride is dried with 200 mg of
anhydrous sodium sulfate. The solution is filtered and the filtrate is
evaporated to yield 25-hydroxycholecalciferol essentially anhydrous as an
amorphous oil.
brand name
Calderol (Organon).
Therapeutic Function
Calcium regulator
General Description
25-Hydroxyvitamin D
3 (calcifediol) is a prohormone produced via hydroxylation of vitamin D
3 (cholecalciferol) in the liver. It is a precursor for the synthesis of calcitriol {1,25-dihydroxyvitamin D
3 or [1,25(OH)
2D
3]}. It is used as a biomarker to determine the status of vitamin D in the body.
25-Hydroxyvitamin D
3-23,24,25,26,27-
13C
5?is an isotope of vitamin D
3 wherein C-23, C-24, C-25, C-26, C-27 carbons are replaced by
13C
6 isotope.
Biochem/physiol Actions
Ergocalciferol (vitamin D2) and 25-Hydroxycholecalciferol (vitamin D3) are the two form of vitamin D which are activated in vivo by hydroxylation. Vitamin D2 and D3 may be used in a wide range of studies to assess their effects on function such as immune function and calcium homeostasis.