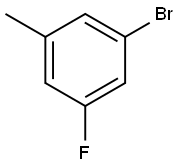
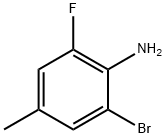
Uses
3-Bromo-5-fluorotoluene is an important halotoluene compound used as a structural component of many organic heterocyclic compounds. It has reactive groups, bromine and fluorine atoms, which can be used in substitution reactions.
Synthesis
2-Bromo-6-fluoro-4-methylaniline (11.2 g, 55 mmol), diluted hydrochloric acid (28.5 mL, 6 M) and acetonitrile (300 mL) were added to a 500 mL single-neck flask, then to the mixture in flask was added dropwise slowly a solution of sodium nitrite (5.69 g, 82.4 mmol) in water (40 mL) at 0 °C. After the addition, the reaction mixture was stirred at 0 °C for 1 h; then a solution of potassium iodide (18.3 g, 110 mmol) was added to the flask in water (30 mL). After the addition, the mixture was stirred at 0 °C for 0.5 h, then stirred at rt overnight. The reaction mixture was added saturated aqueous sodium sulfite (300 mL), and the resulting mixture was concentrated in vacuo to remove acetonitrile. The residue was extracted with ethyl acetate (100 mL x 2), combining the organic layers. The combined organic layers were washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated in vacuo. The residue was purified by silica-gel column chromatography (ethyl acetate/petroleum ether (v/v) = 1/100) to give 1-Bromo-3-fluoro-5-methylbenzene as a white solid (13.2 g, 76%).