Upadacitinib
- Product NameUpadacitinib
- CAS1310726-60-3
- CBNumberCB53121345
- MFC17H19F3N6O
- MW380.37
- EINECS213-161-9
- MDL NumberMFCD30502663
- MOL File1310726-60-3.mol
- MSDS FileSDS
Chemical Properties
| Density | 1.56±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:42.67(Max Conc. mg/mL);112.17(Max Conc. mM) DMSO:PBS (pH 7.2) (1:1):0.5(Max Conc. mg/mL);1.31(Max Conc. mM) DMF:30.0(Max Conc. mg/mL);78.87(Max Conc. mM) Ethanol:76.0(Max Conc. mg/mL);199.8(Max Conc. mM) |
| form | A crystalline solid |
| pka | 11.89±0.60(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C17H19F3N6O/c1-2-10-7-25(16(27)24-9-17(18,19)20)8-11(10)13-5-22-14-6-23-15-12(26(13)14)3-4-21-15/h3-6,10-11,21H,2,7-9H2,1H3,(H,24,27)/t10-,11+/m1/s1 |
| InChIKey | WYQFJHHDOKWSHR-MNOVXSKESA-N |
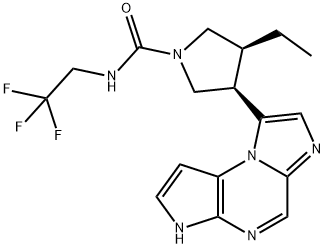
| SMILES | N1(C(NCC(F)(F)F)=O)C[C@H](C2N3C4C=CNC=4N=CC3=NC=2)[C@H](CC)C1 |
| FDA UNII | 4RA0KN46E0 |
| ATC code | L04AA44 |
Upadacitinib Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| Cayman Chemical 29706 | 5mg | $116 | Upadacitinib |
Buy |
| Cayman Chemical 29706 | 25mg | $381 | Upadacitinib |
Buy |
| Cayman Chemical 29706 | 1mg | $32 | Upadacitinib |
Buy |
| Cayman Chemical 29706 | 10mg | $169 | Upadacitinib |
Buy |
| TRC U800075 | 1mg | $300 | Upadacitinib |
Buy |
Upadacitinib Chemical Properties,Usage,Production
Introduction
Upadacitinib (ABT-494) is a Janus kinase 1 (JAK1) inhibitor currently being developed by AbbVie for the treatment of rheumatoid arthritis (RA), Crohn’s disease, ulcerative colitis, atopic dermatitis, and psoriatic arthritis. It is also being investigated as a potential treatment for people with active ankylosing spondylitis (AS).
AS is an inflammatory, progressive autoimmune disease that affects the joints of the spine. As the disease progresses, calcium deposits form where ligaments attach to the bones that form the spine, leading to reduced flexibility of the back.
Description
Upadacitinib is a JAK1 inhibitor (IC50 = 47 nM). It is selective for JAK1 over JAK3 and tyrosine kinase 2 (Tyk2; IC50s = 2,304 and 4,690 nM, respectively), as well as a panel of 83 additional kinases at 1 μM, but does inhibit JAK2, Rho-associated kinase I (ROCK1), and ROCK2 (IC50s = 120, 920, and 430 nM, respectively). Upadacitinib decreases cytokine-induced STAT phosphorylation in a variety of human cells with IC50 values ranging from 1.6 to 649 nM. It reduces M. tuberculosis-induced paw swelling and bone erosion in a rat model of arthritis when administered at doses of 1, 3, and 10 mg/kg twice per day for 17 days. Formulations containing upadacitinib have been used in the treatment of rheumatoid arthritis.Characteristics
Class: non-receptor tyrosine kinaseTreatment: psoriatic arthritis, rheumatoid arthritis
Elimination half-life = 6–16 h
Protein binding = 52%
Uses
Upadacitinib also known as ABT-494, is a potent and selective Janus kinase (JAK) 1 inhibitor being developed for the treatment of several autoimmune disorders, Janus kinase inhibitors for rheumatoid arthritis. It is used to treat moderate to severely active rheumatoid arthritis, active psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis (a type of arthritis in the spine) with objective signs of swelling, moderate to severe ulcerative colitis, and moderate to severe Crohn's disease in patients who have taken other medicines (eg, methotrexate) that did not work well. It is also used to treat moderate to severe atopic dermatitis (eczema) in patients who have taken other medicines that did not work well and whose condition is not well controlled with other treatments or in patients who cannot tolerate these treatments. It is also used to treatpsoriatic arthritis, axial SpA and Giant Cell Arteritis and Takayasu Arteritis.brand name
Upadacitinib is marketed under the brand name RINVOQ for oral administration.Biological Activity
Upadacitinib is a potent, orally active and selective Janus kinase 1 (JAK1) inhibitor (IC50=43 nM). Upadacitinib displays approximately 74 fold selective for JAK1 over JAK2 (200 nM) in cellular assays dependent on specific, relevant cytokines. Upadacitinib can be used for several autoimmune disorders research. In vivo, Upadacitinib inhibited paw swelling and bone destruction in a rat model of arthritis.Mechanism of action
The Janus kinases (JAKs) are a family of cytoplasmic tyrosine kinases whose function is to transduce cytokine-mediated signals via the JAK-STAT pathway. There are four JAK subtypes, each of which has overlapping receptor responsibilities. Inhibitors of this enzyme family (jakinibs) have shown efficacy in treating certain inflammatory and autoimmune diseases such as rheumatoid arthritis and Crohn's disease. However, the first generation of these drugs, tofacitinib and ruxolitinib, lacked subtype selectivity, affecting JAK1/JAK3 and JAK1/JAK2 respectively. This has led to dose-limiting side effects in this otherwise promising class of drugs. Upadacitinib is a second generation Janus kinase inhibitor that is selective for the JAK1 subtype of this enzyme over the JAK2 (74-fold), JAK3 (58-fold) and tyrosine kinase 2 subtypes.Clinical Use
Upadacitinib is indicated for the treatment of moderate to severe active rheumatoid arthritis in adults who have responded inadequately to, or who are intolerant to one or more disease-modifying antirheumatic drugs (DMARDs). Upadacitinib may be used as monotherapy or in combination with methotrexate.Side effects
Common side effects are upper respiratory tract infections such as common cold and sinus infections (13.5% of patients in studies), nausea (3.5%), cough (2.2%), fever, and increased liver enzymes. Serious side effects include infections, including life-threatening ones, such as pneumonia, cellulitis, tuberculosis, as well as shingles and other herpes infections.Synthesis
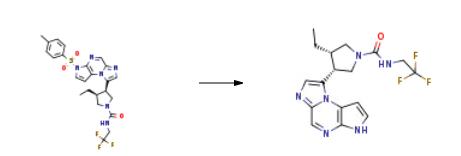
To a solution of (3S,4R)-3-ethyl-4-(3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazin-8-yl)- N-(2,2,2-trifluoroethyl)pyrrolidine-l-carboxamide (10 g) in tetrahydrofurane (50 ml_), a 10% solution of sodium hydroxide in water (15 ml) was added and heated to 50??C. The reaction mixture was stirred for 5 hours and cooled to 25??C. A saturated solution of sodium chloride (100 ml_) was added to the reaction mixture and extracted with dichloromethane (100 ml). The organic phase was separated and washed with water (100 ml_). An 2.5 % aqueous solution of HCI (100 ml) was added and stirred for 30 minutes. The organic phase was removed and the resultant acid aqueous phase was extracted with dichloromethane (100 ml). The final aqueous phase was cooled down to 0/5 ??C and a 10% solution of NaOH was charged until pH 10/12. The resultant suspension was filtered and washed with water (2 x 50 ml_). The cake was drained and dried at 30/40??C under vacuum to give an almost white amorphous solid. Yield: 80%. 1 H NMR (400MHz, d-DMSO) 612.27 (s,1H), 8.58 (s,1H), 7.47-7.43 (m,2H), 7.00-6.94 (m,2H), 4.38 -4.33 (m,1H), 3.92-3.67 (m,5H), 3.33-3.25 (m,1 H), 2.59-2.54 (m,1 H), 1.14-1.08 (m,1 H), 0.86-0.78 (m, 1H), 0.65-0.62 (m, 3H).
Metabolism
Upadacitinib displays a dose-proportional pharmacokinetic profile over the therapeutic dose range. Following oral administration, the median time to reach Cmax (Tmax) ranges from 2 to 4 hours. The steady-state plasma concentrations of upadacitinib are reached within 4 days following multiple once-daily administrations, with minimal accumulation. Food intake has no clinically relevant effect on the AUC, Cmax, and Cmin of upadacitinib from the extended-release formulation. Upadacitinib metabolism is mainly mediated by CYP3A4, with a potential minor contribution from CYP2D6. Administration of strong CYP3A inhibitors increases upadacitinib AUC by 75% and Cmax by 70%, while strong inducers of CYP3A reduce upadacitinib plasma exposures by approximately half[1].References
[1] Mohamed-Eslam F. Mohamed. “Upadacitinib: Mechanism of action, clinical, and translational science.” Cts-Clinical and Translational Science 17 1 (2023).storage
Store at -20°CPreparation Products And Raw materials
Upadacitinib Supplier
| Supplier | Tel | Country | ProdList | Advantage | ||
|---|---|---|---|---|---|---|
| 21-38751876 +8615000076078 |
info@rochipharma.com | China | 431 | 58 | ||
| +86-576-88902229;+86-0576-88902229 +8613968687450 |
yuxin@yuxchem.com | China | 167 | 58 | ||
| +86-0531-69954981 +8615666777973 |
dwyane.wang@boyuanpharm.com | China | 211 | 58 | ||
| +86-010-67886402 +8613611125266 |
market@hopelife.cn | China | 72 | 58 | ||
| +86-18600796368 +86-18600796368 |
sales@sjar-tech.com | China | 485 | 58 | ||
| +8617774091612 | marketing@sybiochem.com | China | 179 | 58 | ||
| +86-020-61855200-902 +8618124244216 |
info@upharm.cn | China | 897 | 58 | ||
| +86-18632776803 +86-13833998158 |
cangzhoukangrui@126.com | China | 739 | 58 | ||
| +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12816 | 58 | ||
| +86-027-59207850 | info@fortunachem.com | China | 5966 | 58 |
Related articles
JAK inhibitor Upadacitinib:FDA approval,Brand name,Indications and Side effects
Upadacitinib received FDA approval on August 16, 2019, based on positive and promising results from its multinational phase III trials in subjects with moderate to severe rheumatoid arthritis.
Mar 24,2025
Upadacitinib: Uses; Mechanism of Action; Administration and Contraindications
Upadacitinib is FDA-approved for the treatment of moderate to severe rheumatoid arthritis (RA) that has not responded to first-line therapy.
Dec 19,2023
Synthesis and Clinical study of Upadacitinib
Upadacitinib, a JAK1 inhibitor, has shown good efficacy in clinical trials for the treatment of a variety of autoimmune and inflammatory diseases.
Oct 18,2022
Upadacitinib Spectrum
1310726-60-3, UpadacitinibRelated Search
- Baricitinib
- Ibrutinib
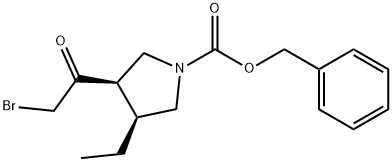
- (3R,4S)-3-(2-Bromoacetyl)-4-ethyl-1-pyrrolidinecarboxylic acid phenylmethyl ester
- tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate
- [2-[(3R,4S)-4-ethyl-1-[(phenylmethoxy)carbonyl]-3-pyrrolidinyl]-2-oxoethyl]dimethyl-Sulfoxonium inner salt
- AZD-9291
- Nintedanib Ethanesulfonate Salt
- Olaparib
- Vandetanib
- 3-(1-Methyl-1H-pyrazol-4-yl)-5-oxo-N-(2-pyridinylmethyl)-5H-benzo[4,5]cyclohepta[1,2-b]pyridine-7-methanesulfonamide
1of4
The What'sApp is temporarily not supported in mainland China