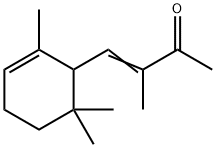
ALPHA-ISO-METHYLIONONE
- Product NameALPHA-ISO-METHYLIONONE
- CAS127-51-5
- CBNumberCB5183650
- MFC14H22O
- MW206.32
- EINECS204-846-3
- MDL NumberMFCD00034582
- MOL File127-51-5.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | 121-122℃ (9 Torr) |
| Density | 0.93 g/mL at 20 °C(lit.) |
| vapor pressure | 0.22Pa at 20℃ |
| refractive index | 1.50188 (589.3 nm 20℃) |
| FEMA | 2714 | ALPHA-ISO-METHYLIONONE |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | liquid |
| color | Light orange to Yellow to Green |
| Odor | at 10.00 % in dipropylene glycol. woody violet floral |
| Odor Type | floral |
| Viscosity | 38.68mm2/s |
| Water Solubility | 27.953mg/L at 25℃ |
| JECFA Number | 404 |
| Stability | Light Sensitive |
| LogP | 4.288 at 25℃ |
| Substances Added to Food (formerly EAFUS) | ALPHA-ISOMETHYLIONONE |
| FDA UNII | 9XP4LC555B |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H411 | |||||||||
| Precautionary statements | P273-P391-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 43 | |||||||||
| Safety Statements | 24/25-36/37 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 2 | |||||||||
| HS Code | 2914.23.0000 | |||||||||
| NFPA 704: |
|


